determination of catalpol in rat plasma and cerebrospinal fluid:application to an in vivo pharmacokinetic study[j].j pharm biomed anal.2012,70:337
‑
43.),在转化成新药之前需要进行结构修饰改造。通过对梓醇类衍生物的结构与生物活性间的文献可以发现,对于梓醇类衍生物其结构变化位置主要在c6
‑
位羟基,而对梓醇c10
‑
位的结构修饰研究未有报道。而且通过对梓醇类抗肿瘤特性所需的全部药效团的组成可以清楚的看到在各种天然产物中加入卤素和杂环基可以增强它们的生物效应。因此对梓醇特定位置引入杂环基和卤素的结构设计合成有重要意义。
技术实现要素:
[0005]
本发明的目的是提供一种新型梓醇衍生物及其制备方法和应用,通过对梓醇c
10
‑
位羟基结构修饰从而得到一种反应条件温和、简单、高效的梓醇衍生物。
[0006]
为达到上述目的,本发明采用的技术方案是:
[0007]
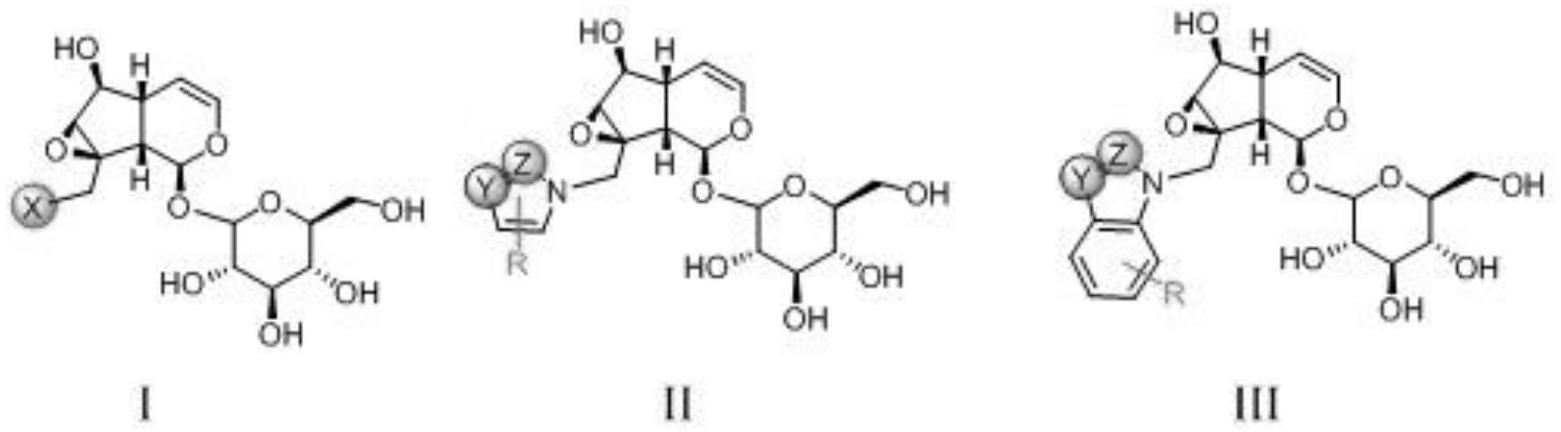
一种梓醇衍生物,所述梓醇衍生物是具有式i、式ii和式iii所示化学结构或/和其可药用衍生物:
[0008][0009]
式i、式ii和式iii中的x、y、z、r为:x为f、cl、br、i,y、z为一个、两个或三个c、n、o和s;r选自氢原子、氘原子、取代或非取代c1‑
c8烷基、取代或非取代氘代c1‑
c8烷基、取代或非取代c2‑
c8烯烃基、取代或非取代c1‑
c8烷氧基、卤素、氨基、硝基、羟基、酰基、氰基、取代或非取代c3‑
c8环烷基、取代或非取代含有1~3个选自n、o、s杂原子的5~8元杂环基、取代或非取代5~8元芳基、取代或非取代含有1~3个选自n、o、s杂原子的5~8元杂芳基;其中所述取代是选自下组中的一个或多个取代基取代:c1‑
c8烷基、卤代c1‑
c8烷基、卤素、氨基、硝基、氰基、羟基、c1‑
c8烷氧基、卤代c1‑
c8烷氧基、羟代c1‑
c8烷基、c3‑
c8环烷基、含有1~3个选自n、o、s杂原子的5~8元杂环基、5~8元芳基、含有1~3个选自n、o、s杂原子的5~8元杂芳基。
[0010]
进一步的,式i、式ii和式iii中的x、y、z、r为:x为f、cl、br、i等卤素,y、z分别独立地选自一个、两个或三个c、n、o和s;r选自取代或未取代c1‑
c8烷氧基、取代或未取代c1‑
c8烷基、取代或未取代c3‑
c8环烷基、取代或未取代含有1~3个选自n、o、s杂原子的5~8元杂环基、取代或非取代5~8元芳基、取代或非取代含有1~3个选自n、o、s杂原子的5~8元杂芳基;其中所述取代是选自下组中的一个或多个取代基取代:c1‑
c8烷基、卤代c1‑
c8烷基、卤素、氨基、硝基、氰基、羟基、羟甲基、羟乙基、巯基、羧基、酯基、c1‑
c6烷基单取代胺基、c1‑
c6烷基双取代胺基、c1‑
c6烷氧基、c1‑
c6烷基羰氧基、c3‑
c6环烷基羰氧基、含有1~3个选自n、o、s杂原子的5~8元杂环基羰氧基、c3‑
c6烷基羰基、c3‑
c6环烷氧基羰基、含有1~3个选自n、o、s杂原子的5~8元杂环氧基羰基、c1‑
c6烷氧甲酰胺基、c1‑
c6烷巯基。
[0011]
进一步的,含氮杂环基或含氮杂芳基是杂环结构或螺环结构或两个杂环直接相连。
[0012]
进一步的,所述通式i、通式ii和通式iii表示的化合物的药学上可接受的盐包括:无机酸盐,有机酸盐,烷基磺酸盐,无机酸盐包括盐酸盐、氢溴酸盐、硝酸盐、硫酸盐、磷酸盐;有机酸盐包括甲酸盐、乙酸盐、丙酸盐、苯甲酸盐、马来酸盐、富马酸盐、琥珀酸盐、酒石酸盐、柠檬酸盐;烷基磺酸盐包括甲基磺酸盐、乙基磺酸盐等;还包括芳基磺酸盐,如苯磺酸盐、对甲苯磺酸盐。
[0013]
进一步的,所述通式i、通式ii和通式iii表示的化合物的药学上可接受的溶剂合物包括通式i、通式ii和通式iii表示的化合物与水、乙醇、异丙醇、乙醚、丙酮的溶剂合物。
[0014]
进一步的,所述通式i、通式ii和通式iii表示的梓醇衍生物具有结构式如下所示:
[0015]
[0016][0017]
一种梓醇衍生物的制备方法,包括以下步骤:
[0018]
(1)化合物a在卤素单质或氢卤化物下发生反应,得到碘代物b;
[0019]
(2)碘代物b与取代杂环反应,获得化合物c和d;
[0020]
具体反应关系式如下:
[0021][0022]
一种药物组合物,所述药物组合物包括通式i、通式ii和通式iii表示的梓醇衍生物、其药学上可接受的盐或药学上可接受的溶剂合物,和任选的药学上可接受的赋形剂。
[0023]
一种药物组合物在制备治疗抗肿瘤药物中的应用。
[0024]
一种药物组合物在制备治疗食管癌细胞和胰腺癌细胞中的应用。
[0025]
本发明具有的优点是:本发明首次探究了在对梓醇c
10
‑
位的结构修饰获得了一种梓醇衍生物,通过对梓醇特定位置引入杂环基和卤素的结构合成一种新的梓醇衍生物,对食管癌细胞和胰腺癌细胞均具有显著抑制作用,在制备新型抗肿瘤药物中具有良好的应用前景,提供的制备方法仅用两步就合成出一系列新型梓醇衍生物,合成方法温和高效,实验证明本发明所述的式(i)、式(ii)和式(iii)化合物或其水合物、药学上可接受的盐或药学上可接受的溶剂合物或所述的药物组合物对食管癌细胞和胰腺癌细胞具有显著的抑制作用,在制备抗肿瘤药物上有着广阔的应用空间。
附图说明
[0026]
图1是化合物ii
‑
4对2株食管癌细胞的ic
50
值测定关系图。
[0027]
图2是化合物ii
‑
1对2株食管癌细胞的ic
50
值测定关系图。
[0028]
图3是化合物ii
‑
9对四株胰腺癌细胞的抑制活性测定关系图。
具体实施方式
[0029]
实施例1
[0030]
一种梓醇衍生物,所述梓醇衍生物是具有式i、式ii和式iii所示化学结构或/和其可药用衍生物:
[0031][0032]
式i、式ii和式iii中的x、y、z、r为:x为f、cl、br、i,y、z为一个、两个或三个c、n、o和s;r选自氢原子、氘原子、取代或非取代c1‑
c8烷基、取代或非取代氘代c1‑
c8烷基、取代或非取代c2‑
c8烯烃基、取代或非取代c1‑
c8烷氧基、卤素、氨基、硝基、羟基、酰基、氰基、取代或非取代c3‑
c8环烷基、取代或非取代含有1~3个选自n、o、s杂原子的5~8元杂环基、取代或非取代5~8元芳基、取代或非取代含有1~3个选自n、o、s杂原子的5~8元杂芳基;其中所述取代是选自下组中的一个或多个取代基取代:c1‑
c8烷基、卤代c1‑
c8烷基、卤素、氨基、硝基、氰基、羟基、c1‑
c8烷氧基、卤代c1‑
c8烷氧基、羟代c1‑
c8烷基、c3‑
c8环烷基、含有1~3个选自n、o、s杂原子的5~8元杂环基、5~8元芳基、含有1~3个选自n、o、s杂原子的5~8元杂芳基。
[0033]
进一步的,式i、式ii和式iii中的x、y、z、r为:x为f、cl、br、i等卤素,y、z分别独立地选自一个、两个或三个c、n、o和s;r选自取代或未取代c1‑
c8烷氧基、取代或未取代c1‑
c8烷基、取代或未取代c3‑
c8环烷基、取代或未取代含有1~3个选自n、o、s杂原子的5~8元杂环基、取代或非取代5~8元芳基、取代或非取代含有1~3个选自n、o、s杂原子的5~8元杂芳基;其中所述取代是选自下组中的一个或多个取代基取代:c1‑
c8烷基、卤代c1‑
c8烷基、卤素、氨基、硝基、氰基、羟基、羟甲基、羟乙基、巯基、羧基、酯基、c1‑
c6烷基单取代胺基、c1‑
c6烷基双取代胺基、c1‑
c6烷氧基、c1‑
c6烷基羰氧基、c3‑
c6环烷基羰氧基、含有1~3个选自n、o、s杂原子的5~8元杂环基羰氧基、c3‑
c6烷基羰基、c3‑
c6环烷氧基羰基、含有1~3个选自n、o、s杂原子的5~8元杂环氧基羰基、c1‑
c6烷氧甲酰胺基、c1‑
c6烷巯基。
[0034]
进一步的,含氮杂环基或含氮杂芳基是杂环结构或螺环结构或两个杂环直接相连。
[0035]
进一步的,所述通式i、通式ii和通式iii表示的化合物的药学上可接受的盐包括:无机酸盐,有机酸盐,烷基磺酸盐,无机酸盐包括盐酸盐、氢溴酸盐、硝酸盐、硫酸盐、磷酸盐;有机酸盐包括甲酸盐、乙酸盐、丙酸盐、苯甲酸盐、马来酸盐、富马酸盐、琥珀酸盐、酒石酸盐、柠檬酸盐;烷基磺酸盐包括甲基磺酸盐、乙基磺酸盐等;芳基磺酸盐,如苯磺酸盐、对甲苯磺酸盐。
[0036]
进一步的,所述通式i、通式ii和通式iii表示的化合物的药学上可接受的溶剂合物包括通式i、通式ii和通式iii表示的化合物与水、乙醇、异丙醇、乙醚、丙酮的溶剂合物。
[0037]
进一步的,所述通式i、通式ii和通式iii表示的梓醇衍生物具有结构式如下所示:
[0038]
[0039][0040]
一种梓醇衍生物的制备方法,包括以下步骤:
[0041]
(1)化合物a在卤素单质或氢卤化物下发生反应,得到卤代物b;
[0042]
(2)卤代物b与取代杂环反应,获得化合物c和d;
[0043]
具体反应关系式如下:
[0044]
[0045]
一种药物组合物,所述药物组合物包括通式i、通式ii和通式iii表示的梓醇衍生物、其药学上可接受的盐或药学上可接受的溶剂合物,和任选的药学上可接受的赋形剂。
[0046]
一种药物组合物在制备治疗抗肿瘤药物中的应用。
[0047]
一种药物组合物在制备治疗食管癌细胞和胰腺癌细胞中的应用。
[0048]
实验例
[0049]
下面实施例中未注明具体条件的实验方法,通常按照常规条件或按照制造厂商所建议的条件。化合物的结构用bruker
‑
500mhz型核磁共振仪测定,氘代二甲基亚砜(dmso)为溶剂,四甲基硅烷(tms)为内标。层析柱一般使用200~300目硅胶为载体。
[0050]
一、化合物制备例部分
[0051]
实验例1:i类化合物的制备
[0052][0053]
化合物b的合成:
[0054]
在反应瓶中加入梓醇36.2mg(0.1mmol)和1ml超干四氢呋喃,0℃冰水浴且氮气保护下依次加入咪唑85.7mg(1.26mmol)、三苯基膦157.4mg(0.6mmol)和碘单质152.3mg(0.6mmol),于0℃反应至终点(tlc跟踪检测)。加入适量200
‑
300目硅胶伴样,减压回收溶剂,经柱层析硅胶v(二氯甲烷):v(甲醇)=15:1,得到化合物b(即i类化合物),收率为:70%。
[0055]
i
‑
1,1h nmr(500mhz,dmso
‑
d6)δ6.37(dd,j=5.9,1.8hz,1h),5.33(s,1h),5.05(s,1h),4.98(dd,j=6.0,4.6hz,2h),4.89(d,j=9.7hz,1h),4.58(d,j=7.4hz,1h),4.11(d,j=10.4hz,1h),3.81
–
3.75(m,1h),3.68(dd,j=11.9,2.0hz,1h),3.66
–
3.62(m,1h),3.38(dd,j=11.8,6.8hz,1h),3.23(d,j=10.4hz,1h),3.21
–
3.10(m,5h),3.03
–
2.95(m,1h),2.36(dd,j=9.7,7.6hz,1h),2.12(m,j=8.0,4.6,1.8hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.80,103.28,99.18,94.33,78.28,77.74,77.19,73.75,70.58,68.89,63.58,61.84,49.07,42.75,37.48.hrms(esi)m/zcalcdforc
15
h
25
nio9[m nh4]
:490.0562,found:490.0565.
[0056]
实验例2:ii类化合物的制备
[0057][0058]
化合物b的合成:
[0059]
在反应瓶中加入梓醇36.2mg(0.1mmol)和1ml超干四氢呋喃,0℃冰水浴且氮气保护下依次加入咪唑85.7mg(1.26mmol)、三苯基膦157.4mg(0.6mmol)和碘单质152.3mg(0.6mmol),于0℃反应至终点(tlc跟踪检测)。加入适量200
‑
300目硅胶伴样,减压回收溶剂,经柱层析硅胶v(二氯甲烷):v(甲醇)=15:1,得到化合物b,收率为:70%。
[0060]
ii类化合物的合成:
[0061]
在反应瓶中将化合物b(1mmol)溶于n,n
‑
二甲基甲酰胺中,在室温搅拌下加入m1‑
r(6.6mmol)和无水碳酸钾(2.2mmol),于70℃反应至终点(tlc跟踪检测)。加入适量200
‑
300目硅胶伴样,减压回收溶剂,经柱层析硅胶分离得到相应的式ii类化合物,收率在50%
‑
90%之间。
[0062]
表1制备新型梓醇衍生物的底物试剂(m1‑
r)
[0063][0064][0065]
ii
‑
1:1h nmr(500mhz,dmso
‑
d6)δ7.68(s,1h),7.22(s,1h),6.95(s,1h),6.38(dd,j=6.0,1.8hz,1h),5.04
–
4.97(m,2h),4.78(d,j=15.0hz,1h),4.65(d,j=7.9hz,1h),4.31
(d,j=15.1hz,5h),3.85
–
3.82(m,1h),3.73(dd,j=11.9,2.1hz,1h),3.48(dd,j=11.9,6.3hz,1h),3.22(q,j=7.4,6.0hz,2h),3.19(s,1h),3.16(s,1h),3.12
–
3.08(m,2h),2.21(s,1h),2.10
–
2.06(m,1h).
13
c nmr(125mhz,dmso
‑
d6)δ168.28,165.13,163.79,140.83,103.27,99.92,94.85,77.71,77.16,76.92,73.78,70.36,63.40,63.21,61.62,49.04,42.01,37.78.hrms(esi )calculated for c
18
h
25
n2o9[m h]
:413.1554,found:413.1556。
[0066]
ii
‑
2:1h nmr(500mhz,dmso
‑
d6)δ7.10(s,1h),6.67(s,1h),6.39(d,j=5.9hz,1h),5.38(s,1h),5.19(dd,j=24.4,10.0hz,3h),5.05(d,j=9.7hz,1h),5.01
–
4.98(m,1h),4.64(dd,j=7.9,1.9hz,1h),4.46(d,j=7.9hz,2h),3.82(d,j=8.2hz,1h),3.71(d,j=11.7hz,1h),3.45(d,j=6.7hz,1h),3.25
–
3.15(m,3h),3.07(dd,j=21.9,8.9hz,2h),2.94(s,1h),2.34(t,j=8.7hz,1h),2.23(d,j=1.9hz,3h),2.10(q,j=7.0hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ145.41,140.86,126.34,121.12,103.38,99.46,94.50,77.90,77.12,77.00,73.82,70.55,63.80,62.32,61.83,44.52,42.64,37.86,13.21.hrms(esi )calculated for c
19
h
27
n2o9[m h]
:427.1711,found:427.1709。
[0067]
ii
‑
3:1h nmr(500mhz,dmso
‑
d6)δ6.80(d,j=1.2hz,1h),6.39(dd,j=6.0,1.7hz,1h),5.49
–
5.06(m,3h),5.05
–
4.97(m,3h),4.64(d,j=7.9hz,1h),4.37(d,j=4.4hz,2h),3.81(d,j=8.2hz,1h),3.73(dd,j=11.8,2.0hz,1h),3.43(dd,j=11.8,6.9hz,2h),3.21(t,j=8.9hz,2h),3.08(t,j=8.5hz,1h),3.03(d,j=9.1hz,1h),2.98(s,1h),2.34(dd,j=9.7,7.4hz,1h),2.18(s,3h),2.11(ddt,j=9.4,4.9,2.3hz,1h),2.00(s,3h).
13
c nmr(150mhz,dmso
‑
d6)δ144.62,140.89,134.23,117.22,103.37,99.44,94.50,77.92,77.19,77.01,73.81,63.81,62.41,61.94,49.06,44.43,42.59,37.87,13.83,13.03.hrms(esi )calculated for c
20
h
27
n2o9[m h]
:441.1867,found:441.1864。
[0068]
ii
‑
4:1h nmr(500mhz,dmso
‑
d6)δ7.77(d,j=7.7hz,2h),7.64(d,j=15.7hz,2h),7.35(t,j=7.6hz,2h),7.20(t,j=7.4hz,1h),6.40(dd,j=5.9,1.7hz,1h),5.36(d,j=5.6hz,1h),5.27(d,j=5.1hz,1h),5.10
–
4.98(m,4h),4.81(d,j=15.1hz,1h),4.68(t,j=7.6hz,2h),4.34(d,j=15.1hz,1h),3.85(dd,j=8.5,3.7hz,1h),3.81
–
3.74(m,1h),3.53(dd,j=11.4,6.3hz,1h),3.24(d,j=10.0hz,3h),3.19
–
3.10(m,2h),2.31(dd,j=9.7,7.4hz,1h),2.17
–
2.09(m,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.87,140.47,139.09,134.83,128.96,126.72,124.71,116.95,103.29,100.00,94.91,77.76,77.25,76.99,73.89,70.41,63.45,63.25,61.71,46.64,42.04,37.83.hrms(esi )calculated for c
24
h
29
n2o9[m h]
:489.1867,found:489.1865。
[0069]
ii
‑
5:1h nmr(500mhz,dmso
‑
d6)δ7.54(d,j=1.5hz,1h),7.25(d,j=1.5hz,1h),6.39(dd,j=6.0,1.7hz,1h),5.35(d,j=5.6hz,1h),5.24(d,j=5.2hz,1h),5.06(d,j=4.9hz,1h),5.04
–
4.97(m,3h),4.78(d,j=15.1hz,1h),4.64(d,j=7.8hz,1h),4.60(dd,j=6.9,4.9hz,1h),4.26(d,j=15.0hz,1h),3.83(dd,j=8.3,5.4hz,1h),3.73(m,j=12.0,6.8,2.0hz,1h),3.48(m,j=11.5,5.6hz,1h),3.25
–
3.18(m,3h),3.09(m,j=17.1,8.9,4.7hz,2h),2.25(dd,j=9.7,7.5hz,1h),2.10(m,j=7.7,4.6,1.8hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.85,138.83,119.75,113.91,103.24,100.06,94.88,77.70,77.18,76.92,73.78,70.32,63.31,63.18,61.62,47.02,41.94,37.78.hrms(esi )calculated for c
18
h
24
brn2o9[m h]
:491.0659,found:491.0656。
[0070]
ii
‑
6:1h nmr(500mhz,dmso
‑
d6)δ8.33(d,j=1.5hz,1h),8.02(d,j=1.5hz,1h),7.20(dd,j=6.0,1.8hz,1h),6.16(d,j=5.6hz,1h),6.05(d,j=5.2hz,1h),5.92
–
5.84(m,1h),5.84
–
5.78(m,3h),5.58(d,j=15.1hz,1h),5.46(d,j=7.9hz,1h),5.41(dd,j=7.0,4.9hz,1h),5.05(d,j=15.1hz,1h),4.64(dd,j=8.3,5.4hz,1h),4.54(m,j=11.9,6.7,2.1hz,1h),4.33
–
4.25(m,1h),4.04
–
3.96(m,3h),3.90(m,j=13.3,4.3hz,2h),3.07(dd,j=9.7,7.5hz,1h),2.91(m j=7.9,4.6,1.8hz,1h).
13
c nmr(150mhz,dmso
‑
d6)δ140.86,137.65,127.70,116.51,103.24,100.05,94.87,77.71,77.20,76.93,73.78,70.32,63.32,63.16,61.63,47.12,41.93,37.78.hrms(esi )calculated for c
18
h
24
cln2o9[m h]
:447.1164,found:447.1167。
[0071]
ii
‑
7:1h nmr(500mhz,dmso
‑
d6)δ7.52(d,j=1.3hz,1h),7.29(d,j=1.3hz,1h),6.37(dd,j=6.0,1.7hz,1h),5.34(d,j=5.7hz,1h),5.22(d,j=5.2hz,1h),5.05(d,j=5.1hz,1h),5.02
–
4.97(m,3h),4.78(d,j=15.1hz,1h),4.63(d,j=7.9hz,1h),4.59(dd,j=7.0,4.9hz,1h),4.26(d,j=15.0hz,1h),3.82(dd,j=8.3,5.7hz,1h),3.72(m,j=11.8,7.0,2.1hz,1h),3.50
–
3.43(m,1h),3.21(q,j=5.1,4.6hz,3h),3.12
–
3.03(m,2h),2.23(dd,j=9.7,7.5hz,1h),2.09(m,j=9.6,4.7,2.9hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.84,140.77,125.89,103.24,100.06,94.89,82.28,77.70,77.18,76.93,73.79,70.33,63.27,61.64,49.08,46.67,41.95,37.78.hrms(esi )calculated for c
18
h
24
in2o9[m h]
:539.0520,found:539.0518。
[0072]
ii
‑
8:1h nmr(500mhz,dmso
‑
d6)δ7.38(d,j=1.5hz,1h),6.93(d,j=1.4hz,1h),6.40(dd,j=6.0,1.8hz,1h),5.48
–
4.74(m,6h),4.69
–
4.47(m,4h),3.81(dd,j=8.1,1.3hz,1h),3.73(dd,j=11.9,2.0hz,1h),3.45(dd,j=11.8,6.9hz,1h),3.21(m,j=11.3,6.4,5.8,3.2hz,2h),3.11
–
3.00(m,2h),2.98(s,1h),2.39(dd,j=9.8,7.5hz,1h),2.13(m,j=8.0,4.6,1.8hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.91,129.21,124.48,120.46,103.33,99.36,94.27,77.85,77.18,76.95,73.75,70.56,62.98,62.28,61.93,46.25,42.82,37.87.hrms(esi )calculated for c
18
h
24
brn2o9[m h]
:491.0659,found:491.0657。
[0073]
ii
‑
9:1h nmr(500mhz,dmso
‑
d6)δ7.75(s,1h),6.40(dd,j=6.0,1.7hz,1h),5.33(d,j=5.3hz,1h),5.18(d,j=5.0hz,1h),5.12
–
4.92(m,4h),4.67
–
4.58(m,3h),4.54(d,j=21.3hz,1h),3.82(d,j=8.1hz,1h),3.73(d,j=11.7hz,1h),3.49
–
3.39(m,2h),3.20(m,j=10.3,5.9,2.9hz,2h),3.05(q,j=10.4,10.0hz,2h),2.39(dd,j=9.7,7.5hz,1h),2.17
–
2.09(m,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.94,137.14,124.28,113.82,103.25,99.54,94.36,77.82,77.14,76.90,73.72,70.46,62.70,62.10,61.85,49.06,42.71,37.84.hrms(esi )calculated for c
18
h
23
cl2n2o9[m h]
:481.0775,found:481.0773。
[0074]
ii
‑
10:1h nmr(500mhz,dmso
‑
d6)δ6.38(dd,j=5.9,1.8hz,1h),5.09
–
4.96(m,4h),4.93(d,j=9.7hz,1h),4.58(d,j=7.7hz,1h),4.36(d,j=10.9hz,1h),3.81(d,j=8.3hz,1h),3.69(dd,j=11.8,2.0hz,1h),3.59(d,j=1.1hz,1h),3.44(d,j=10.9hz,1h),3.42
–
3.37(m,1h),3.27
–
3.19(m,1h),3.19
–
3.12(m,3h),3.09(dd,j=9.0,7.8hz,1h),3.01(q,j=9.3,8.5hz,1h),2.42(dd,j=9.8,7.5hz,1h),2.11(m,j=7.9,4.6,1.8hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.87,103.25,99.21,94.22,77.74,77.72,77.15,73.80,70.57,
66.79,63.16,62.43,61.82,61.18,49.08,41.94,37.53,35.75.hrms(esi )calculated for c
18
h
22
br3n2o9[m h]
:648.8849,found:648.8852。
[0075]
ii
‑
11:1h nmr(500mhz,dmso
‑
d6)δ7.54(d,j=1.3hz,1h),7.16(d,j=1.2hz,1h),6.39(dd,j=6.0,1.8hz,1h),5.33(d,j=5.7hz,1h),5.15(d,j=5.2hz,1h),5.10(d,j=15.3hz,1h),5.06
–
5.01(m,2h),5.01
–
4.96(m,3h),4.59(d,j=7.9hz,1h),4.54(dd,j=7.1,5.0hz,1h),3.79(m,j=7.4,5.8,1.3hz,1h),3.73(m,j=11.8,7.1,2.1hz,1h),3.54
–
3.46(m,1h),3.17(d,j=5.2hz,2h),3.05(m,j=9.2,5.4hz,1h),3.01
–
2.93(m,1h),2.90(d,j=1.3hz,1h),2.40(dd,j=9.8,7.6hz,1h),2.13(m,j=8.1,4.6,1.8hz,1h).
13
c nmr(150mhz,dmso
‑
d6)δ145.90,140.88,127.91,127.87,103.23,99.78,94.44,77.64,77.17,76.86,73.63,70.24,62.77,61.84,61.69,48.04,42.98,37.95.hrms(esi )calculated for c
18
h
24
n3o
11
[m h]
:458.1405,found:458.1407。
[0076]
ii
‑
12:1h nmr(500mhz,dmso
‑
d6)δ9.50(s,1h),7.25(t,j=2.0hz,1h),6.97(dd,j=4.0,1.7hz,1h),6.40(dd,j=6.0,1.8hz,1h),6.23(dd,j=4.1,2.4hz,1h),5.39
–
5.11(m,2h),5.10
–
5.02(m,3h),5.00(dd,j=5.9,4.5hz,1h),4.85(d,j=15.3hz,1h),4.66(d,j=7.9hz,1h),4.62
–
4.32(m,1h),3.78
–
3.70(m,2h),3.45(dd,j=11.9,6.7hz,2h),3.22(m,j=11.1,6.5,5.7,3.2hz,2h),3.10
–
3.00(m,2h),2.73(d,j=1.1hz,1h),2.37(dd,j=9.7,7.6hz,1h),2.12(m,j=7.9,4.6,1.9hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ180.17,140.79,132.79,132.72,109.89,103.38,99.47,94.40,77.67,77.31,76.96,73.82,70.55,64.01,62.16,61.93,55.37,46.11,43.01,37.97.hrms(esi )calculated for c
20
h
26
no
10
[m h]
:440.1551,found:440.1553。
[0077]
ii
‑
13:1h nmr(500mhz,dmso
‑
d6)δ7.68(d,j=2.3hz,1h),7.41(d,j=1.9hz,1h),6.40(d,j=6.0hz,1h),5.27(d,j=5.4hz,1h),5.18(d,j=5.1hz,1h),5.01(m,j=15.0,5.1hz,4h),4.93(d,j=15.2hz,1h),4.76(m,j=6.6,2.9hz,1h),4.70(d,j=15.2hz,1h),4.66(d,j=7.9hz,1h),3.78(dd,j=8.2,5.6hz,1h),3.77
–
3.70(m,1h),3.48(dt,j=12.0,6.0hz,2h),3.20(m,j=9.7,5.0hz,3h),3.14
–
3.06(m,2h),2.35(dd,j=9.8,7.4hz,1h),2.12(q,j=7.4hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.97,138.86,131.76,105.72,103.30,99.97,95.01,77.51,77.28,76.96,73.78,70.27,63.48,61.95,61.49,50.34,42.77,37.87.hrms(esi )calculated for c
18
h
25
n2o9[m h]
:413.1554,found:413.1556。
[0078]
ii
‑
14:1h nmr(500mhz,dmso
‑
d6)δ7.50(d,j=2.1hz,1h),6.38(dd,j=5.9,1.8hz,1h),6.00(d,j=2.2hz,1h),5.23(d,j=5.4hz,1h),5.17(d,j=5.2hz,1h),5.04
–
4.97(m,5h),4.84(t,j=6.7hz,1h),4.65(dd,j=7.9,1.6hz,1h),4.52(d,j=15.4hz,1h),3.78
–
3.71(m,2h),3.50(m,j=12.1,6.2hz,1h),3.24
–
3.15(m,3h),3.15
–
3.06(m,2h),2.33(dd,j=9.8,7.4hz,1h),2.13(s,4h).
13
c nmr(150mhz,dmso
‑
d6)δ147.35,141.01,132.58,105.27,103.27,100.23,95.25,77.37,77.29,76.93,73.79,70.13,63.67,61.80,61.30,49.79,42.76,37.92,13.53.hrms(esi )calculated for c
19
h
27
n2o9[m h]
:427.1711,found:427.1711。
[0079]
ii
‑
15:1h nmr(500mhz,dmso
‑
d6)δ7.89(s,1h),7.52(s,1h),6.39(dd,j=6.0,1.7hz,1h),5.29(d,j=5.5hz,1h),5.15(d,j=5.1hz,1h),5.04
–
4.97(m,4h),4.78(d,j=15.2hz,1h),4.70(d,j=15.2hz,1h),4.63(d,j=7.8hz,1h),4.55(dd,j=6.9,5.5hz,1h),
3.81(m,j=8.1,5.5,1.3hz,1h),3.72(m,j=11.9,7.1,2.1hz,1h),3.47(dt,j=11.9,5.9hz,1h),3.19(m,j=9.0,5.9,2.5hz,2h),3.11
–
3.02(m,2h),2.34(dd,j=9.7,7.4hz,1h),2.11(m,j=9.7,7.8,4.7,1.8hz,1h),1.44
–
1.17(m,3h),0.87(t,j=7.3hz,1h).
13
c nmr(150mhz,dmso
‑
d6)δ140.94,139.34,131.79,103.31,99.80,94.76,92.21,77.65,77.24,76.95,73.78,70.31,62.97,62.11,61.60,51.54,42.70,37.83,14.38.hrms(esi )calculated for c
19
h
27
n2o9[m h]
:427.1711,found:427.1711。
[0080]
ii
‑
16:1h nmr(500mhz,dmso
‑
d6)δ7.49(d,j=2.2hz,1h),6.38(dd,j=6.0,1.8hz,1h),6.00(d,j=2.2hz,1h),5.24
–
5.16(m,2h),5.04
–
4.97(m,5h),4.96(s,1h),4.84(q,j=6.3,5.8hz,1h),4.64(d,j=7.8hz,1h),4.52(d,j=15.4hz,1h),3.75(m,j=12.4,10.5,6.0hz,2h),3.49(m,j=11.6,5.6hz,1h),3.19(m,j=7.9,2.2hz,2h),3.13
–
3.06(m,2h),2.70(s,1h),2.33(dd,j=9.8,7.4hz,1h),2.12(s,3h).
13
c nmr(150mhz,dmso
‑
d6)δ147.35,141.01,132.58,105.27,103.27,100.22,95.24,77.36,77.28,76.92,73.79,70.13,63.66,61.81,61.30,49.80,42.76,37.91,13.54.hrms(esi )calculated for c
19
h
27
n2o9[m h]
:427.1711,found:427.1711。
[0081]
ii
‑
17:1h nmr(500mhz,dmso
‑
d6)δ6.39(dd,j=5.9,1.9hz,1h),5.24(s,1h),5.21(s,1h),5.16(d,j=7.8hz,1h),5.01
–
4.93(m,3h),4.65(d,j=7.9hz,1h),4.31(d,j=15.9hz,1h),3.79
–
3.70(m,2h),3.49(dd,j=12.5,5.9hz,1h),3.20(m,j=8.2,2.6hz,2h),3.13
–
3.06(m,2h),2.52(s,3h),2.46(s,1h),2.40(dd,j=9.8,7.4hz,1h),2.35(s,1h),2.16(s,3h),2.06(s,3h).
13
c nmr(125mhz,dmso
‑
d6)δ146.73,141.39,141.08,104.94,103.27,100.44,95.48,77.34,77.21,76.97,73.80,70.17,64.50,61.25,61.14,46.25,43.18,37.97,13.45,11.34.hrms(esi )calculated for c
19
h
27
n2o9[m h]
:441.1867,found:441.1867。
[0082]
ii
‑
18:1h nmr(500mhz,dmso
‑
d6)δ7.73(d,j=2.3hz,1h),6.42
–
6.37(m,2h),5.30(d,j=5.5hz,1h),5.15(d,j=5.2hz,1h),5.02(dd,j=10.5,5.5hz,4h),4.79(d,j=15.3hz,1h),4.66
–
4.60(m,2h),4.56
–
4.50(m,1h),3.83(dd,j=8.4,5.4hz,1h),3.73(m,j=11.9,7.0,2.1hz,1h),3.48(m,j=11.8,5.7hz,1h),3.20(m,j=9.8,6.4,2.6hz,2h),3.11
–
3.05(m,3h),2.33(dd,j=9.7,7.5hz,1h),2.13(m,j=9.6,7.6,4.6,1.8hz,1h).
13
c nmr(150mhz,dmso
‑
d6)δ140.91,134.59,124.42,108.47,103.33,99.66,94.66,77.71,77.24,76.94,73.78,70.35,62.86,62.15,61.63,51.49,42.70,37.80.hrms(esi )calculated for c
18
h
24
brn2o9[m h]
:491.0659,found:491.0655。
[0083]
ii
‑
19:1h nmr(500mhz,dmso
‑
d6)δ7.95
–
7.74(m,1h),7.57
–
7.38(m,1h),6.39(dd,j=5.9,1.7hz,1h),5.30(d,j=5.5hz,1h),5.17(dd,j=10.6,5.1hz,1h),5.01(m,j=9.9,6.6,4.3hz,4h),4.76
–
4.60(m,3h),4.57(q,j=6.2hz,1h),3.81(dd,j=8.6,5.3hz,1h),3.73(m,j=12.0,7.0,2.1hz,1h),3.47(dt,j=11.8,5.8hz,1h),3.20(m,j=6.5,3.6hz,2h),3.08(m,j=9.3,5.3hz,2h),2.99(d,j=5.0hz,1h),2.35(dd,j=9.7,7.5hz,1h),2.16
–
2.08(m,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.94,131.79,125.91,117.96,103.31,99.72,94.72,77.63,77.24,76.94,73.77,70.32,63.03,62.15,61.58,51.99,42.65,37.82.hrms(esi )calculated for c
18
h
24
fn2o9[m h]
:431.1460,found:431.1462。
[0084]
ii
‑
20:1h nmr(500mhz,dmso
‑
d6)δ7.89(s,1h),7.51(s,1h),6.38(dd,j=5.9,
1.8hz,1h),5.29(s,1h),5.15(s,1h),5.00(dt,j=10.6,4.0hz,4h),4.76(d,j=15.2hz,1h),4.70
–
4.61(m,2h),4.55(q,j=13.6,12.1hz,1h),3.81(d,j=8.2hz,1h),3.72(d,j=11.8hz,1h),3.46(dd,j=11.9,6.1hz,1h),3.22
–
3.17(m,2h),3.06(m,j=8.9,5.1hz,2h),2.99(d,j=1.1hz,1h),2.34(dd,j=9.7,7.5hz,1h),2.11(m,j=7.8,4.6,1.7hz,1h).
13
c nmr(150mhz,dmso
‑
d6)δ140.94,137.26,129.75,108.50,103.31,99.77,94.74,77.64,77.23,76.94,73.77,70.31,62.94,62.13,61.59,51.66,42.68,37.82.hrms(esi )calculated for c
18
h
24
cln2o9[m h]
:447.1164,found:447.1167。
[0085]
ii
‑
21:1h nmr(500mhz,dmso
‑
d6)δ7.89(s,1h),7.52(s,1h),6.39(dd,j=6.1,1.8hz,1h),5.28(t,j=6.2hz,1h),5.17
–
5.10(m,1h),5.09
–
4.93(m,4h),4.79(d,j=15.2hz,1h),4.70(d,j=15.2hz,1h),4.64(d,j=7.8hz,1h),4.55(d,j=6.5hz,1h),3.81(dd,j=8.6,3.7hz,1h),3.75
–
3.68(m,1h),3.47(d,j=17.8hz,1h),3.24
–
3.13(m,2h),3.07(q,j=8.3hz,2h),2.98(s,1h),2.34(dd,j=9.8,7.5hz,1h),2.15
–
2.07(m,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.93,139.33,131.78,103.30,99.78,94.76,92.20,77.62,77.21,76.94,73.77,70.30,62.96,62.11,61.58,51.54,42.70,37.81.hrms(esi )calculated for c
18
h
24
brn2o9[m h]
:491.0659,found:491.0656。
[0086]
ii
‑
22:1h nmr(500mhz,dmso
‑
d6)δ7.84(s,1h),7.50(s,1h),6.39(dd,j=6.0,1.7hz,1h),5.29(d,j=5.5hz,1h),5.15(d,j=5.2hz,1h),5.04
–
4.98(m,4h),4.83
–
4.72(m,2h),4.64(d,j=7.8hz,1h),4.57(t,j=6.3hz,1h),3.81(dd,j=8.3,5.4hz,1h),3.73(m,j=12.1,7.0,2.2hz,1h),3.47(m,j=11.7,5.8hz,1h),3.20(m,j=9.4,6.0,3.6hz,2h),3.07(m,j=13.8,9.0,5.2hz,2h),2.94(s,1h),2.34(dd,j=9.7,7.5hz,1h),2.12(m,j=7.7,4.8,1.7hz,1h).
13
c nmr(150mhz,dmso
‑
d6)δ143.84,140.94,135.92,103.30,99.84,94.79,77.63,77.24,76.96,73.79,70.30,63.10,62.07,61.60,57.44,51.14,42.73,37.84.hrms(esi )calculated for c
18
h
24
in2o9[m h]
:539.0521,found:539.0519。
[0087]
ii
‑
23:1h nmr(500mhz,dmso
‑
d6)δ8.71(s,1h),8.24(s,1h),6.38(dd,j=5.9,1.7hz,1h),5.34(d,j=5.6hz,1h),5.13
–
4.88(m,7h),4.66
–
4.60(m,2h),4.54(t,j=6.1hz,1h),3.85(dd,j=8.3,5.2hz,1h),3.76
–
3.69(m,1h),3.53
–
3.48(m,1h),3.20(dt,j=9.8,2.8hz,2h),3.11(dd,j=9.3,3.9hz,1h),3.02(m,j=8.5,3.8hz,1h),2.37(dd,j=9.7,7.5hz,1h),2.13(m,j=7.6,4.7,1.8hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.87,135.79,135.36,132.00,103.23,99.90,94.72,77.66,77.23,76.90,73.67,70.17,62.23,61.94,61.53,49.06,42.72,37.77.hrms(esi )calculated for c
18
h
24
n3o
11
[m h]
:458.1405,found:458.1405。
[0088]
ii
‑
24:1h nmr(500mhz,dmso
‑
d6)δ7.55(s,1h),6.40(dd,j=5.9,1.8hz,1h),5.31(d,j=5.8hz,1h),5.12
–
4.93(m,7h),4.87(d,j=15.7hz,1h),4.60(d,j=7.9hz,1h),4.52(dd,j=7.9,4.7hz,1h),3.91
–
3.85(m,1h),3.72(m,j=11.9,7.7,2.1hz,1h),3.42(m,j=11.7,7.1,4.6hz,1h),3.18(m,j=7.4,2.7hz,2h),3.04(s,1h),2.98(m,j=8.1,5.1,3.0hz,2h),2.14(m,j=7.9,4.5,1.9hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.97,140.50,133.95,122.04,120.51,107.51,103.34,98.84,93.74,77.97,77.07,76.90,73.75,70.59,61.97,61.74,61.69,50.93,43.88,37.82.hrms(esi )calculated for c
20
h
23
f6n2o9[m h]
:571.1122,found:571.1121。
[0089]
ii
‑
25:1h nmr(500mhz,dmso
‑
d6)δ7.97
–
7.93(m,1h),6.70(d,j=2.3hz,1h),6.39(dd,j=6.1,1.8hz,1h),5.41
–
5.23(m,1h),5.16
–
4.97(m,4h),4.94(d,j=15.2hz,1h),4.68
–
4.62(m,2h),4.52(s,1h),3.85(d,j=8.1hz,1h),3.72(dd,j=12.0,2.1hz,1h),3.47(dd,j=11.9,6.3hz,2h),3.20(m,j=8.8,2.1hz,2h),3.11(s,1h),3.10
–
3.03(m,2h),2.35(dd,j=9.7,7.5hz,1h),2.11(m,j=7.7,4.7,1.8hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.88,134.00,120.95,104.68,103.32,99.58,94.53,77.76,77.20,76.93,73.75,70.37,62.61,62.32,61.68,51.89,49.06,42.71,37.80.hrms(esi )calculated for c
19
h
24
f3n2o9[m h]
:481.1428,found:481.1426。
[0090]
ii
‑
26:1h nmr(500mhz,dmso
‑
d6)δ8.27(s,1h),7.87(s,1h),6.39(dd,j=6.0,1.8hz,1h),5.31(d,j=5.6hz,1h),5.11(d,j=5.1hz,1h),5.01(m,j=11.2,5.5,4.9hz,4h),4.87(d,j=15.2hz,1h),4.75(d,j=15.2hz,1h),4.64(d,j=7.8hz,1h),4.55(t,j=6.2hz,1h),3.83(dd,j=8.2,5.4hz,1h),3.73(m,j=11.9,6.9,2.0hz,1h),3.49(mj=11.5,5.7hz,1h),3.24
–
3.17(m,3h),3.07(m,j=16.2,8.8,4.8hz,2h),2.36(dd,j=9.7,7.5hz,1h),2.13(m,j=7.5,4.6,1.8hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ140.91,136.76,131.85,112.08,103.26,99.86,94.76,77.65,77.23,76.95,73.77,70.24,62.73,62.02,61.57,51.35,49.06,42.78,37.82.hrms(esi )calculated for c
19
h
24
f3n2o9[m h]
:481.1428,found:481.1424。
[0091]
实验例3:iii类化合物的制备
[0092][0093]
化合物b的合成:
[0094]
在反应瓶中加入梓醇36.2mg(0.1mmol)和1ml超干四氢呋喃,0℃冰水浴且氮气保护下依次加入咪唑85.7mg(1.26mmol)、三苯基膦157.4mg(0.6mmol)和碘单质152.3mg(0.6mmol),于0℃反应至终点(tlc跟踪检测)。加入适量200
‑
300目硅胶伴样,减压回收溶剂,经柱层析硅胶v(二氯甲烷):v(甲醇)=15:1,得到化合物b,收率为:70%。
[0095]
iii类化合物的合成:
[0096]
在反应瓶中将化合物b(1mmol)溶于n,n
‑
二甲基甲酰胺中,在室温搅拌下加入m2‑
r(6.6mmol)和无水碳酸钾(2.2mmol),于70℃反应至终点(tlc跟踪检测)。加入适量200
‑
300目硅胶伴样,减压回收溶剂,经柱层析硅胶分离得到相应的式iii类化合物,收率在50%
‑
90%之间。
[0097]
表2制备新型梓醇衍生物的底物试剂(m2‑
r)
[0098][0099]
iii
‑
1:1h nmr(500mhz,dmso
‑
d6)δ9.15(s,1h),8.90(s,1h),8.55(s,1h),6.41(dd,j=6.0,1.8hz,1h),5.29(d,j=5.6hz,1h),5.19(d,j=5.1hz,1h),5.08(d,j=9.7hz,1h),5.05
–
4.96(m,4h),4.87(d,j=15.4hz,1h),4.68(d,j=7.8hz,1h),4.53(t,j=6.3hz,1h),3.86
–
3.81(m,1h),3.81
–
3.74(m,1h),3.56(m,j=11.9,5.9hz,1h),3.23(m,j=17.3,8.9,5.2hz,2h),3.13
–
3.01(m,2h),3.00(s,1h),2.48
–
2.43(m,1h),2.14(m,j=7.5,3.5hz,1h).
13
c nmr(125mhz,dmso
‑
d6)δ152.45,151.97,148.30,147.80,140.96,133.62,103.32,99.72,94.59,77.65,77.22,76.88,73.72,70.29,62.75,62.08,61.67,43.02,42.27,37.93.hrms(esi )calculated for c
20
h
25
n4o9[m h]
:465.1616,found:465.1616。
[0100]
iii
‑
2:1h nmr(500mhz,dmso
‑
d6)δ8.14(s,1h),7.67(d,j=8.0hz,1h),7.63(d,j=7.9hz,1h),7.25(t,j=7.5hz,1h),7.20(t,j=7.5hz,1h),6.41(d,j=6.0hz,1h),5.27(d,j=5.5hz,1h),5.21(d,j=5.1hz,1h),5.15(d,j=9.7hz,1h),5.06(t,j=4.6hz,2h),5.00(t,j=5.4hz,1h),4.91(d,j=15.7hz,1h),4.77(d,j=15.7hz,1h),4.71(d,j=8.2hz,2h),3.81(m,j=11.4,5.8hz,2h),3.53(m,j=11.4,6.9,4.0hz,1h),3.31
–
3.21(m,2h),3.09(m,j=18.3,9.2,8.7,4.2hz,2h),2.87(s,1h),2.37(dd,j=9.7,7.5hz,1h),2.13(t,j=6.6hz,1h).
13
c nmr(150mhz,dmso
‑
d6)δ145.16,143.26,140.98,134.97,122.76,121.97,119.63,111.54,103.28,99.80,94.76,78.08,77.19,77.03,73.80,70.57,63.42,62.35,61.95,43.81,42.61,37.84.hrms(esi )calculated for c
22
h
27
n2o9[m h]
:463.1711,found:463.1711。
[0101]
iii
‑
3:1h nmr(500mhz,dmso
‑
d6)δ8.18(d,j=157.9hz,1h),7.86
–
7.46(m,2h),7.43
–
6.93(m,2h),6.44
–
6.38(m,1h),5.29
–
4.97(m,8h),4.95
–
4.86(m,1h),4.69(dd,j=7.8,3.3hz,1h),4.46(d,j=5.3hz,2h),3.80(m,j=13.8,8.1,4.9hz,2h),3.53(m,j=15.8,8.9,4.7hz,1h),3.30
–
3.17(m,2h),3.17
–
3.08(m,1h),3.05(m,j=8.3,7.8,4.3hz,1h),2.18
–
2.10(m,1h).
13
c nmr(125mhz,dmso
‑
d6)δ141.03,140.66,133.23,126.42,123.64,121.08,117.24,110.87,103.38,99.66,94.79,77.85,77.30,77.02,73.78,70.50,63.93,63.26,61.51,47.25,43.40,37.90.hrms(esi )calculated for c
22
h
27
n2o9[m h]
:463.1711,found:463.1711。
[0102]
二、体外抗肿瘤抑制活性测试部分
[0103]
实验例4:式i、ii类化合物对四株食管癌细胞的抑制作用
[0104]
实验利用来源、病理分型明确,对药物敏感的eca109、ec9706、kyse150和kyse70四株食管癌细胞,在mtt培养板上观察合成目标化合物在体外对细胞直接的生长抑制或杀伤
作用。
[0105]
实验作用时间:24h,48h
[0106]
通过对4株食管癌细胞筛选,最终确定有六种化合物对四株食管癌细胞具有生长抑制作用,其中进一步测定化合物ii
‑
4和ii
‑
1对两株食管癌细胞的ic
50
值。
[0107]
表3四种化合物对2株食管癌细胞细胞存活率
[0108][0109]
表4两种化合物对2株食管癌细胞ic
50
值
[0110][0111]
化合物ii
‑
4、化合物ii
‑
1两种化合物对2株食管癌细胞的生长抑制作用图见图1和图2所示。
[0112]
实验例5:式ii、iii类化合物对3株胰腺癌细胞的抑制作用
[0113]
对合成的30个梓醇衍生物,选择3株不同的人胰腺癌细胞aspc
‑
1、bxpc
‑
3和panc
‑
1并同时选择人正常胰腺导管上皮细胞hpde6
‑
c7进行毒性评估试验。
[0114]
试验方法:panc
‑
1细胞使用dmem培养基培养,另加入10%fbs,100u/ml青霉素和100μg/ml链霉素,aspc
‑
1、bxpc
‑
3和hpde6
‑
c7细胞使用1640培养基培养,另加入10%fbs,100u/ml青霉素和100μg/ml链霉素。各细胞系均于含5%co2的37℃恒温培养箱中培养。待细胞生长状态良好对其进行计数,并以每孔2000
‑
4000个细胞接种于96孔板中,待细胞贴壁(约24h)后,加入不同浓度或种类化合物进行处理。37℃恒温培养箱作用一定时间后,每孔加入10μl mtt(5mg/ml),置于培养箱中继续孵育4h。随后,吸出培养基和mtt,每孔分别加入150μl dmso。将培养板放置于摇床上震荡15
‑
30min,至甲臜完全溶解。然后用酶标仪在490nm波长处检测每孔的吸光度(optical density,od)。实验中要设置调零孔(培养基、mtt、二甲基亚砜)。
[0115]
细胞存活率=(处理组od
‑
调零孔od)/(对照组od
‑
调零孔od)
×
100%
[0116]
结果化合物ii
‑
9对胰腺癌的抑制效果呈时间依赖性(图3),且对不同种胰腺癌细
胞特别是bxpc
‑
3具有良好的抑制活性,化合物ii
‑
9刺激72h后,对aspc
‑
1细胞的抑制率为78.0%,bxpc
‑
3细胞的抑制率为91.6%,panc
‑
1细胞的抑制率为73.1%。且对正常胰腺导管上皮细胞也有一定抑制作用,抑制率低于胰腺癌细胞为62.5%(原因是由于长期培养条件下,hpde6
‑
c7细胞某些基因突变可无限增殖,使其具有肿瘤细胞特性)。
[0117]
本发明的优点一是不同于以往梓醇衍生物的合成方法,本发明的制备方法为首次制备合成。根据文献调研与总结,发现近二十年对梓醇不同位置进行修饰的研究已有报道。如carlos r.pungitore课题组和celina garc
í
a课题组进行了梓醇部分硅醚化研究,carlos e.tonn课题组对梓醇进行了先硅醚化后酯化以及全酯化的探索,carlos r.pungitore课题组对梓醇结构进行了全酯化的探索。上海中医药大学张刘强等对6
‑
位酯化的梓醇衍生物梓苷和胡黄连苷ii进行结构修饰,得到一系列对8
‑
羟基鸟嘌呤dna糖基化酶1(ogg1)具有显著抑制活性的梓醇衍生物。
[0118][0119]
通过对已报道梓醇结构修饰的研究进展可知,前期对梓醇修饰主要集中在对梓醇的部分或全部硅醚化以及部分或全部酯化研究而对梓醇羟基定向引入药效团未见报道。对于梓醇类衍生物其结构变化主要梓苷的c6‑
位羟基,而对梓醇c
10
‑
位羟基结构修饰研究未有报道。
[0120]
本发明的优点二,实验证明本发明所述的式(i)、式(ii)和式(iii)化合物或其水合物、药学上可接受的盐或药学上可接受的溶剂合物或所述的药物组合物对肿瘤细胞具有显著的抑制作用,在制备抗肿瘤药物上有着广阔的应用空间。
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。