1.本发明属于化学合成技术领域,涉及苯并氮杂环类化合物及其制备方法,本发明的苯并氮杂环类化合物包括1-苄基异吲哚和1-苄基四氢异喹啉化合物。
背景技术:
2.苯并氮杂环类化合物如,1-苄基异吲哚和1-苄基四氢异喹啉骨架广泛存在于多种天然产物或药物中,具有多种生物活性,主要包括抗菌、抗炎、抗肿瘤、抗氧化、降血压、调节免疫等功能
1.。例如,从暗罗属细基丸的根部中分离得到的劳丹素(laudanosine)
2.和从小檗属植物中分离得到的lennoxamine
3.都具有潜在的抗肿瘤、抗神经精神病、血管舒张,gaba受体和镇咳作用
4.;从罂粟花中分离得到的诺司卡品(noscapine)
5.是一种镇咳剂
[6a]
,在治疗中风、焦虑和癌症方面显示出潜在的临床用途
[6]
;从罂粟科紫堇属植物延胡索中分离得到的原小檗碱生物碱canadine具有钙通道阻滞剂的作用
[7]
。因此,含1-苄基异吲哚和1-苄基四氢异喹啉骨架结构的天然产物的研究近年来颇受化学家和药理学家的偏爱,但在这类骨架的快速、高效合成方法学及普适的多样性合成方面均留有很大的研究空间。
[0003][0004]
对于1-苄基异吲哚和1-苄基四氢异喹啉骨架的合成,文献报道的方法中,1-取代异吲哚可通过氨基烯烃的分子内氢胺化、meyers方法(通过其叔丁基甲酰胺衍生物的金属化和烷基化从2,3-二氢-1h-异吲哚合成)和异吲哚-1,3-二酮的加成-还原-消除-还原;其中,只有第一种方法报道了1-苄基异吲哚啉的合成,尽管已有这些合成方法,但一个更加直接、有效的制备1-苄基异吲哚的方法仍然是需要的。对于1-取代四氢异喹啉,文献报道的方法很多,包括卤代酰胺环化、pictet-spengler反应、阳极制备的α-氨基腈烷基化、bia级联、和ir催化的1-取代异喹啉氢化反应,然而,所述方法中存在反应条件苛刻或适应性差的缺
点,直接有效地合成1-苄基异喹啉的方法仍然十分有限,因此,发展新的合成方法以实现苯并氮杂环类化合物如1-苄基异吲哚和1-苄基四氢异喹啉骨架的多样性大量合成,可以为新药研究提供物质基础。
[0005]
与本发明相关的参考文献有:
[0006]
[1](a)p.m.dewick,medicinal natural products:a biosynthetic approach,wiley,chichester,2002,p.315;(b)bentley,k.w.,β-phenylethylamines and the isoquinoline alkaloids.nat.prod.rep.2006,23,444-463;(c)sovic,i.;karminski-zamola,g.,derivatives of isoindoline,synthesis and biological activity.ii.biological activity of isoindoline derivatives.kem.ind.2014,63,183-191.
[0007]
[2]s.kanokmedhakul,k.kanokmedhakul,r.lekphrom,j.nat.prod.2007,70,1536-1538.
[0008]
[3]a.couture,e.deniau,p.grandclaudon,c.hoarau,tetrahedron 2000,56,1491-1499.
[0009]
[4](a)w.cui,k.iwasa,h.tokuda,a.kashihara,y.mitani,t.hasegawa,y.nishiyama,m.moriyasu,h.nishino,m.hanaoka,c.mukai,k.takeda,phytochemistry 2006,67,70-79;(b)m.asencio,c.hurtado-guzm
á
n,j.j.l
ó
pez,b.k.cassels,p.protais,a.chagraoui,bioorg.med.chem.,2005,13,3699-3704;(c)y.katz,a.weizman,c.g.pick,g.w.pasternak,l.liu,o.fonia,m.gavish,brain res.,1994,646,235-241;(d)f.s.sadritdinov,a.g.kurmukov,pharmacology of plant alkaloids and their use in medicine[in russian];fan:tashkent,1980.
[0010]
[5]p.-j.robiquet,ann.chim.phys.1817,5,275-278.
[0011]
[6](a)y.ke,k.ye,h.e.grossniklaus,d.r.archer,h.c.joshi,j.a.kapp,cancer immunol.,immun.,2000,49,217-225;(b)p.khodarahmi,p.rostami,a.rashidi,i.khodarahmi,pharmacol.rep.,2006,58,568-570.(c)m.mahmoudian,m.mehrpour,f.benaissa,z.siadatpour,eur.j.clin.pharmacol.,2003,59,579-581.
[0012]
[7]s.yang,y.miao,q.han,m.jiang,g.jin,zhongguo yaoli xuebao 1993,14,235-237.。
技术实现要素:
[0013]
本发明的目的在于提供苯并氮杂环类化合物及其制备方法。本发明的苯并氮杂环类化合物包括1-苄基异吲哚和1-苄基四氢异喹啉化合物,所述的1-苄基异吲哚和1-苄基四氢异喹啉化合物可以通过简单转化,得到laudanosine等天然产物。
[0014]
本发明的合成路线的特点是:反应条件温和、简单,可以进行大量制备。
[0015]
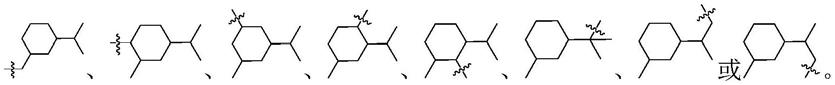
本发明合成的苯并氮杂环类化合物是1-苄基异吲哚和1-苄基四氢异喹啉化合物,其具有式(i)的化学结构:
[0016][0017]
其中,取代基r代表氢、烷基、烷氧基、氰基、酯基、卤原子、砜;r’代卤原子、甲氧基。
[0018]
进一步地,所述1-苄基异吲哚和1-苄基四氢异喹啉化合物具体化学结构如下:
[0019][0020]
本发明按下述技术路线合成包括式(1,2)结构的苯并氮杂环类化合物,所述的式(1,2)结构化合物是1-苄基异吲哚和1-苄基四氢异喹啉化合物,本发明在下文的陈述实施例中,中间体通式是根据结构式中的编号,用阿拉伯数字表示。
[0021][0022]
上述合成路线包括以下步骤:
[0023]
步骤1:氩气保护、室温下将n,o-缩醛化合物3或4和一种路易斯酸溶于一种干燥的有机溶剂中,加入新制的不同苄基取代溴化锌试剂,反应温度升至70度反应1小时后加入饱和碳酸氢钠水溶液,用乙酸乙酯萃取,浓缩,纯化得到目标化合物1a-1q、1ba-1fa和2a-2m;
[0024]
其中一种路易斯酸是指三甲基氯硅烷、三氟化硼乙醚、三氟甲磺酸三甲基硅酯、氯化锌、三氟甲磺酸铜、三氟甲磺酸钪,特别是指三氟甲磺酸钪;
[0025]
一种有机溶剂是指二氯甲烷、三氯甲烷、四氢呋喃、2-甲基四氢呋喃、乙醚、甲苯、苯等,特别是指二氯甲烷和四氢呋喃。
[0026]
本发明所述制备1-苄基异吲哚和1-苄基四氢异喹啉化合物的技术路线,操作简单,路线简洁,收率较高,所用的试剂均为常用试剂,而且,可适合大规模制备,所得目标产物可用于多个具有重要生理活性天然产物的多样性合成研究。
具体实施方式
[0027]
实施例1
[0028]
氩气保护、室温下将n,o-缩醛化合物3或4(0.5mmol)和三氟甲磺酸钪(0.1mmol,49mg)溶于干燥的四氢呋喃中,加入新制的不同苄基取代溴化锌试剂(2.0ml,1m in thf),反应温度升至70度反应1小时后加入饱和碳酸氢钠水溶液,用乙酸乙酯萃取(20ml
×
3),浓缩,纯化得到目标化合物1a-1q、1ba-1fa和2a-2m。
[0029]
合成化合物1a
[0030]
yellow oil(133mg,86%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.21-7.15(m,3h),7.14-7.09(m,2h),7.07-6.97(m,1h),6.96-6.81(m,3h),5.36-5.31(m,0.45h),5.28-5.20(m,0.55h),4.63(d,j=14.8hz,0.55h),4.49(d,j=14.4hz,0.45h),4.12(d,j=14.8hz,0.55h),3.93(d,j=14.8hz,0.45h),3.36-3.28(m,0.45h),3.26-3.19(m,1h),3.12-3.04(m,0.55h),1.59(s,4.95h),1.54(s,4.05h)ppm;.
[0031]
合成化合物1b
[0032]
yellow oil(146mg,90%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.27-7.16(m,2h),7.14-7.04(m,4h),6.96-6.91(m,1h),6.57-6.53(m,0.4h),6.50-6.44(m,0.6h),5.35-5.29(m,0.4h),5.26-5.20(m,0.6h),4.78(d,j=14.8hz,0.6h),4.63(d,j=14.8hz,0.4h),4.47(d,j=14.8hz,0.6h),4.34(d,j=14.8hz,0.4h),3.53-3.48(m,0.4h),3.38-3.30(m,0.6h),2.95-2.87(m,0.4h),2.86-2.79(m,0.6h),2.21-2.18(m,3h),1.54(s,3.61h),1.51(s,5.42h)ppm.
[0033]
合成化合物1c
[0034]
colourless oil(144mg,89%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.23-7.14(m,2h),7.13-7.12(m,0.52h),7.09-7.03(m,1h),7.02-6.93(m,2h),6.86-6.81
(m,0.48h),6.78-6.75(m,1h),6.75-6.71(m,0.52h),6.67-6.62(m,0.48h),5.35-5.27(m,0.48h),5.25-5.20(m,0.52h),4.65(d,j=14.8hz,0.52h),4.49(d,j=14.8hz,0.48h),4.16(d,j=14.8hz,0.52h),3.97(d,j=14.8hz,0.48h),3.24-3.20(m,1h),3.20-3.18(m,0.48h),3.02-2.95(m,0.52h),2.24-2.19(m,3h),1.58(s,4.68h),1.55(s,4.32h)ppm.
[0035]
合成化合物1d
[0036]
colourless oil(134mg,83%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.23-7.16(m,2h),7.15-7.10(m,0.55h),7.06-7.02(m,0.45h),6.99-6.95(m,1.45h),6.95-6.90(m,1h),6.88-6.84(m,0.55h),6.82-6.76(m,2h),5.32-5.17(m,1h),4.63(d,j=14.8hz,0.55h),4.49(d,j=14.4hz,0.45h),4.12(d,j=14.8hz,0.55h),3.97(d,j=14.4hz,0.45h),3.29-3.15(m,1.45h),3.06-2.99(m,0.55h),2.29-2.24(m,3h),1.59(s,4.95h),1.54(s,4.05h)ppm.
[0037]
合成化合物1e
[0038]
colourless oil(156mg,95%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.24-7.09(m,4h),6.97-6.84(m,4h),5.41-5.36(m,0.38h),5.33-5.27(m,0.62h),4.69(d,j=14.8hz,0.62h),4.55(d,j=14.8hz,0.38h),4.25(d,j=14.8hz,0.62h),4.13(d,j=14.8hz,0.38h),3.39-3.32(m,0.38h),3.29-3.20(m,1h),3.16-3.10(m,0.62h),1.54(s,9h)ppm.
[0039]
合成化合物1f
[0040]
light-yellow oil(138mg,84%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.25-7.17(m,2h),7.17-7.10(m,1h),7.07-7.02(m,1.52h),6.91-6.90(m,0.52h),6.87-6.77(m,1.52h),6.74-6.69(m,0.48h),6.64-6.57(m,1.52h),5.37-5.30(m,0.52h),5.27-5.21(m,0.48h),4.65(d,j=14.8hz,0.48h),4.49(d,j=14.4hz,0.52h),4.14(d,j=15.2hz,0.48h),3.95(d,j=14.4hz,0.52h),3.42-3.36(m,0.48h),3.23-3.15(m,1h),3.12-3.06(m,0.52h),1.58(s,4.41h),1.54(s,4.59h)ppm.
[0041]
合成化合物1g
[0042]
colourless oil(138mg,84%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.25-7.18(m,2h),7.15-7.07(m,0.51h),7.07-7.02(m,1h),6.96-6.90(m,0.49h),6.85-6.77(m,4h),5.35-5.29(m,0.49h),5.25-5.20(m,0.51h),4.62(d,j=14.8hz,0.51h),4.48(d,j=14.8hz,0.5h),4.05(d,j=14.8hz,0.49h),3.90(d,j=14.8hz,0.52h),3.41-3.34(m,0.49h),3.15-3.11(m,1.51h),1.59(s,4.51h),1.54(s,4.59h)ppm.
[0043]
合成化合物1h
[0044]
colourless oil(145mg,77%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.68-7.67(m,0.7h),7.63-7.59(m,0.3h),7.47-7.42(m,1.3h),7.39-7.35(m,0.7h),7.31-7.26(m,1h),7.26-7.21(m,1h),7.20-7.17(m,1h),7.15-7.10(m,0.7h),7.07-7.03(m,0.3h),6.73-6.68(m,0.7h),6.49-6.45(m,0.3h),5.47-5.40(m,0.3h),5.37-5.32(m,0.7h),4.90(d,j=15.2hz,0.7h),4.71(d,j=15.2hz,0.3h),4.63-4.57(m,1h),3.68-3.50(m,0.3h),3.24-3.15(m,0.7h),3.14-3.05(m,1h),1.49(s,2.7h),1.29(s,7.3h)ppm.
[0045]
合成化合物1i
[0046]
colourless oil(128mg,68%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ
7.44-7.36(m,1h),7.32-7.25(m,1h),7.23-7.15(m,2h),7.13-7.08(m,2h),7.03-6.99(m,0.58h),6.97-6.92(m,1h),5.41-5.35(m,0.58h),5.30-5.25(m,0.42h),4.62(d,j=14.8hz,0.42h),4.45(d,j=14.8hz,0.58h),4.01(d,j=14.4hz,0.42h),3.79(d,j=14.8hz,0.58h),3.58-3.52(m,0.58h),3.23-3.13(m,1.42h),1.59(s,3.78h),1.54(s,5.22h)ppm.
[0047]
合成化合物1j
[0048]
colourless oil(153mg,81%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.43-7.34(m,2h),7.27-7.22(m,2h),7.15-6.94(m,4h),5.40-5.35(m,0.53h),5.31-5.26(m,0.47h),4.64(d,j=14.8hz,0.47h),4.49(d,j=14.8hz,0.53h),4.05(d,j=14.8hz,0.47h),3.91(d,j=14.8hz,0.53h),3.50-3.42(m,0.53h),3.25-3.19(m,1.47h),1.58(s,4.22h),1.54(s,4.78h)ppm.
[0049]
合成化合物1k
[0050]
colourless oil(139mg,81%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.29-7.18(m,2.46h),7.17-7.09(m,2h),7.07-7.04(m,1h),7.03-6.99(m,0.54h),6.93-6.89(m,1h),6.84-6.80(m,0.46h),6.70-6.66(m,0.54h),5.36-5.29(m,0.54h),5.26-5.21(m,0.46h),4.66(d,j=14.8hz,0.46h),4.49(d,j=14.4hz,0.54h),4.14(d,j=14.4hz,0.46h),3.92(d,j=14.8hz,0.54h),3.42-3.35(m,0.54h),3.18-3.11(m,1h),3.10-3.04(m,0.46h),1.58(s,4.14h),1.55(s,4.86h)ppm.
[0051]
合成化合物1l
[0052]
colourless oil(154mg,84%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.24-7.14(m,4.6h),7.09-7.04(m,0.4h),6.92-6.83(m,3h),5.32-5.28(m,0.4h),5.24-5.19(m,0.6h),4.66(d,j=14.8hz,0.6h),4.52(d,j=14.8hz,0.4h),4.16(d,j=14.8hz,0.6h),4.02(d,j=14.8hz,0.4h),3.27-3.14(m,1.4h),3.04-2.97(m,0.6h),1.56(s,5.4h),1.54(s,3.6h),1.29-1.26(m,9h)ppm.
[0053]
合成化合物1m
[0054]
colourless oil(132mg,79%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.44-7.36(m,2h),7.27-7.21(m,2h),7.15-7.01(m,2h),6.98-6.93(m,2h),5.40-5.27(m,1h),4.61(d,j=15.2hz,0.42h),4.48(d,j=14.4hz,0.58h),3.98(d,j=14.8hz,0.42h),3.86(d,j=14.4hz,0.58h),3.58-3.47(m,0.58h),3.34-3.25(m,0.42h),3.21-3.13(m,1h),1.59(s,3.76h),1.54(s,5.22h)ppm.
[0055]
合成化合物1n
[0056]
white solid(150mg,90%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.63-7.51(m,1h),7.47-7.42(m,1h),7.35-7.27(m,2h),7.24-7.20(m,2h),7.18-7.14(m,1h),7.08-7.02(m,1h),5.45-5.39(m,1h),4.76(d,j=15.2hz,0.55h),4.62(d,j=14.8hz,0.45h),4.33-4.25(m,1h),3.50-3.42(m,1h),3.42-3.38(m,0.45h),3.32-3.27(m,0.55h),1.52(s,4.05h),1.46(s,4.95h)ppm.
[0057]
合成化合物1o
[0058]
colourless oil(153mg,80%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.87-7.82(m,1h),7.81-7.77(m,1h),7.25-7.16(m,2h),7.15-7.08(m,0.46h),7.07-7.02
(m,1h),7.00-6.95(m,1h),6.95-6.92(m,1h),6.92-6.87(m,0.54h),5.40-5.34(m,0.54h),5.31-5.25(m,0.46h),4.62(d,j=14.8hz,0.46h),4.48(d,j=14.4hz,0.54h),4.37-4.30(m,2h),4.05(d,j=14.4hz,0.46h),3.89(d,j=14.8hz,0.54h),3.50-3.44(m,0.54h),3.26-3.21(m,1h),3.21-3.18(m,0.46h),1.60(s,4.14h),1.55(s,4.86h),1.41-1.34(m,3h)ppm.
[0059]
合成化合物1p
[0060]
whitefoam(144mg,80%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ8.52-8.48(m,0.40h),8.38-8.31(m,0.60h),7.92-7.82(m,1h),7.80-1.72(m,1h),7.57-7.53(m,0.40h),7.52-7.50(m,1h),7.49-7.45(m,0.60h),7.39-7.26(m,1h),7.24-7.10(m,2h),7.02-6.94(m,2h),6.36-6.29(m,0.40h),6.20-6.15(m,0.60h),5.55-5.48(m,0.40h),5.47-5.41(m,0.60h),4.82(d,j=14.8hz,0.6h),4.62(d,j=14.4hz,0.4h),4.55(d,j=14.4hz,0.6h),4.32(d,j=14.8hz,0.4h),4.13-4.07(m,0.4h),3.97-3.91(m,0.6h),3.23-2.93(m,1h),1.56(s,9h)ppm.
[0061]
合成化合物1q
[0062]
whitefoam(111mg,62%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.86-7.74(m,2h),7.69-7.65(m,1h),7.61-7.57(m,0.50h),7.45-7.37(m,4h),7.23-7.18(m,1h),7.17-7.14(m,0.50h),7.13-7.09(m,1h),7.04-6.97(m,1.50h),6.87-6.82(m,0.50h),5.47-5.40(m,0.50h),5.36-5.31(m,0.50h),4.65(d,j=14.8hz,0.50h),4.48(d,j=14.8hz,0.50h),4.13(d,j=15.2hz,0.50h),3.91(d,j=15.2hz,0.50h),3.47-3.38(m,1h),3.25-3.16(m,1h),1.61(s,4.50h),1.56(s,4.50h)ppm.
[0063]
合成化合物1ba
[0064]
colourless oil(173mg,89%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.36-7.31(m,1h),7.22-7.13(m,3h),7.09-7.01(m,1h),6.97-6.88(m,2h),6.87-6.83(m,0.50h),6.72-6.67(m,0.50h),5.42-5.37(m,0.5h),5.32-5.28(m,0.5h),4.66(d,j=15.6hz,0.5h),4.47(d,j=15.2hz,0.5h),4.12(d,j=15.2hz,0.5h),3.92(d,j=15.2hz,0.5h),3.25-3.20(m,1.5h),3.05-2.96(m,0.5h),1.58(s,4.49h),1.55(s,4.51h)ppm.
[0065]
合成化合物1ca
[0066]
colourless oil(173mg,89%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.34-7.29(m,1h),7.18-7.17(m,1h),7.16-7.11(m,2h),7.02-6.97(m,1h),6.97-6.84(m,3h),5.31-5.27(m,0.43h),5.23-5.17(m,0.57h),4.55(d,j=15.2hz,0.57h),4.41(d,j=14.8hz,0.43h),4.00(d,j=14.8hz,0.57h),3.83(d,j=14.8hz,0.43h),3.36-3.30(m,0.44h),3.18-3.14(m,1h),3.11-3.04(m,0.57h),1.58(s,5.13h),1.54(s,3.87h)ppm.
[0067]
合成化合物1da
[0068]
colourless oil(156mg,91%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.19-7.12(m,4h),7.11-6.98(m,1h),6.93-6.72(m,3h),5.31-5.23(m,0.47h),5.22-5.15(m,0.53h),4.60(d,j=14.8hz,0.53h),4.45(d,j=14.8hz,0.47h),4.09(d,j=14.8hz,0.53h),3.91(d,j=14.8hz,0.47h),3.31-3.24(m,0.43h),3.24-3.16(m,1h),3.08-3.00(m,0.57h),1.59(s,4.77h),1.54(s,4.23h)ppm.
[0069]
合成化合物1ea
[0070]
colourless oil(129mg,76%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.21-7.10(m,4h),6.99-6.90(m,2h),6.72-6.65(m,1h),6.60-6.53(m,0.55h),6.46-6.40(m,0.45h),5.35-5.27(m,0.55h),5.25-5.19(m,0.55h),4.62(d,j=14.8hz,0.45h),4.47(d,j=14.8hz,0.55h),4.08(d,j=14.8hz,0.45h),3.92(d,j=14.8hz,0.55h),3.77(s,3h),3.27-3.20(m,1.55h),3.07-3.00(m,0.45h),1.58(s,4.05h),1.54(s,4.95h)ppm.
[0071]
合成化合物1fa
[0072]
colourless oil(143mg,84%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.20-7.13(m,3h),7.03-6.93(m,3h),6.77-6.73(m,1h),6.45-6.40(m,0.47h),6.33-6.28(m,0.53h),5.31-5.24(m,0.47h),5.21-5.16(m,0.53h),4.57(d,j=14.4hz,0.53h),4.43(d,j=14.0hz,0.47h),4.09(d,j=14.0hz,0.53h),3.92(d,j=14.0hz,0.47h),3.72-3.68(m,3h),3.26-3.21(m,1.47h),3.05-2.96(m,0.53h),1.59(s,5.13h),1.54(s,3.87h)ppm.
[0073]
合成化合物2a
[0074]
whitefoam(137mg,85%);1h nmr(400mhz,dmso-d6
,80℃)δ7.50-6.90(m,9h),5.36-5.14(m,1h),3.94(brs,1h),3.36-3.26(m,1h),3.07-3.02(m,2h),2.84-2.66(m,2h),1.26(s,9h)ppm;1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.31-7.26(m,1h),7.25-7.23(m,0.7h),7.23-7.19(m,1h),7.19-7.11(m,4h),7.11-7.03(m,2h),6.91-6.86(m,0.3h),5.37(dd,j=7.2,6.4hz,0.3h),5.22(dd,j=8.4,5.6hz,0.7h),4.25-4.17(m,0.7h),3.83-3.75(m,0.3h),3.37-3.33(m,0.3h),3.32-3.24(m,0.7h),3.09-2.97(m,2h),2.97-2.87(m,0.7h),2.84-2.76(m,0.3h),2.74-2.67(m,0.7h),2.65-2.57(m,0.3h),1.40(s,2.7h),1.21(s,6.3h)ppm.
[0075]
合成化合物2b
[0076]
whitefoam(147mg,87%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.19-6.98(m,6h),6.95-6.83(m,2h),5.35(dd,j=7.2,6.4hz,0.28h),5.21(dd,j=8.8,5.2hz,0.72h),4.26-4.17(m,0.72h),3.85-3.75(m,0.28h),3.38-3.35(m,0.28h),3.33-3.25(m,0.72h),3.07-2.96(m,2h),2.95-2.87(m,0.72h),2.84-2.77(m,0.28h),2.75-2.68(m,0.72h),2.66-2.58(m,0.28h),2.32(s,2h),2.28(s,1h),1.41(s,2.52h),1.20(s,6.48h)ppm.
[0077]
合成化合物2c
[0078]
whitefoam(138mg,81%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.24-7.21(m,1h),7.20-7.18(m,2h),7.17-7.12(m,2h),7.09-6.99(m,3h),5.45(dd,j=8.8,5.6hz,0.24h),5.34(dd,j=10.4,4hz,0.76h),4.33-4.25(m,0.76h),3.95-3.87(m,0.24h),3.46-3.40(m,0.24h),3.35-3.26(m,0.76h),3.20-3.13(m,1h),3.09-3.03(m,0.24h),3.02-2.97(m,0.76h),2.96-2.91(m,0.76h),2.86-2.80(m,0.24h),2.77-2.67(m,1h),1.33(s,2.16h),1.15(s,6.84h)ppm.
[0079]
合成化合物2d
[0080]
whitefoam(145mg,74%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.53-7.25(m,4h),7.21-7.08(m,3h),7.07-7.00(m,0.64h),6.93-6.86(m,0.36h),5.37(dd,j=7.2,6.4hz,0.36h),5.21(dd,j=8.4,5.6hz,0.64h),4.28-4.12(m,0.64h),3.85-3.72(m,0.36h),3.38-3.27(m,1h),3.15-3.03(m,2h),3.02-2.77(m,1h),2.76-2.69(m,0.64h),
7.16(m,2h),7.16-7.13(m,3h),7.13-7.09(m,2h),7.09-7.06(m,0.66h),6.95-6.91(m,0.34h),5.37(dd,j=7.6,6.8hz,0.34h),5.20(dd,j=8.4,5.6hz,0.66h),4.27-4.19(m,0.66h),3.86-3.78(m,0.34h),3.38-3.34(m,0.36h),3.31-3.23(m,0.64h),3.09-3.01(m,2h),2.98-2.91(m,0.64h),2.85-2.78(m,0.36h),2.75-2.67(m,0.64h),2.64-2.59(m,0.36h),1.37(s,3.06h),1.20(s,5.94h)ppm.
[0093]
合成化合物2k
[0094]
whitefoam(140mg,78%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.24-7.17(m,4h),7.17-7.09(m,1h),6.90-6.79(m,2h),5.52(dd,j=5.6,4.8hz,0.24h),5.38(dd,j=10.8,4hz,0.76h),4.32-4.23(m,0.76h),4.15-3.95(m,0.24h),3.50-3.43(m,0.24h),3.42-3.31(m,0.76h),3.25-3.13(m,1h),3.11-3.01(m,1h),3.00-2.91(m,0.76h),2.90-2.82(m,0.24h),2.79-2.71(m,1h),1.28(s,2.16h),1.15(s,6.84h)ppm.
[0095]
合成化合物2l
[0096]
whitefoam(115mg,64%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.21-7.11(m,3h),7.07-6.98(m,0.63h),6.96-6.90(m,0.37h),6.73-6.58(m,3h),5.34(dd,j=7.2,6.4hz,0.37h),5.22(dd,j=8.4,5.6hz,0.63h),4.24-4.15(m,0.63h),3.89-3.77(m,0.37h),3.37-3.32(m,0.37h),3.29-3.18(m,0.63h),3.06-2.67(m,4h),1.41(s,3.33h),1.28(s,5.67h)ppm.
[0097]
合成化合物2m
[0098]
whitefoam(151mg,75%);1h nmr(400mhz,cdcl3,mixture of rotamers)δ7.89-7.85(m,1h),7.84-7.77(m,1h),7.36-7.33(m,1h),7.33-7.27(m,1h),7.21-7.11(m,3h),7.07-6.92(m,1h),5.40(dd,j=7.2,6.4hz,0.44h),5.25(dd,j=6.4,5.6hz,0.56h),4.25-4.16(m,0.56h),3.87-3.78(m,0.44h),3.40-3.25(m,1h),3.18-3.11(m,2h),3.04-3.01(m,3h),2.96-2.79(m,1h),2.76-2.63(m,1h),1.38(s,3.96h),1.22(s,5.04h)ppm.。
[0099]
实施例2
[0100]
化合物1b-q,1ba-1fa、2a-2m的制备方法与实施例1相同。
[0101]
合成化合物1a
[0102]
氩气保护、室温下将n,o-缩醛化合物3(0.5mmol)和三氟甲磺酸铜(0.5mmol,181mg)溶于干燥的四氢呋喃中,加入新制的不同苄基取代溴化锌试剂(2.0ml,1m in thf),反应温度升至70度反应1小时后加入饱和碳酸氢钠水溶液,用乙酸乙酯萃取(20ml
×
3),浓缩,纯化得到目标化合物1a(201mg,65%)。
[0103]
实施例3
[0104]
化合物1b-q,1ba-1fa、2a-2m的制备方法与实施例1相同。
[0105]
合成化合物1a
[0106]
氩气保护、室温下将n,o-缩醛化合物3(0.5mmol)和氯化锌(0.5mmol,1m in thf)溶于干燥的四氢呋喃中,加入新制的不同苄基取代溴化锌试剂(2.0ml,1m in thf),反应温度升至70度反应1小时后加入饱和碳酸氢钠水溶液,用乙酸乙酯萃取(20ml
×
3),浓缩,纯化得到目标化合物1a(108mg,35%)。
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。