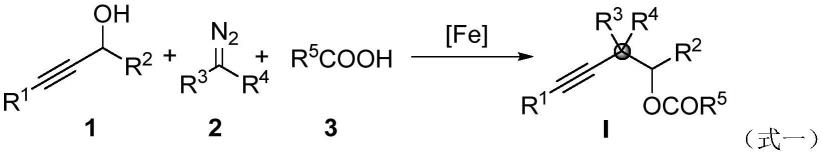
铁催化重氮化合物选择插入炔丙醇的c(sp)
–
c(sp3)键构建季碳中心的方法
技术领域
1.本发明属于有机合成领域,涉及季碳中心的构建方法,具体涉及一种铁催化重氮化合物选择性插入炔丙醇的c(sp)
–
c(sp3)键构建炔烃取代的季碳中心化合物的方法。
背景技术:
2.季碳中心具有刚性和结构多样性,是许多天然产物、药物和生物活性分子的关键结构单元。因此,季碳中心的构建对有机合成化学家具有相当的吸引力,特别是含炔取代框架的季碳中心在有机转化中是通用的中间和基本结构基序。获得炔取代季碳中心最常见的方法是将炔基全部组装到其他底物中,如sonogashira偶联、亲电炔基化、对映选择性偶联炔基化等。上述转变的主要障碍包括末端炔的二聚化、对功能化前驱体的依赖和支链叔烷基单元的β-h消除。在这种情况下,作为解决这些问题的替代策略,本发明人设想是否可以通过选择性裂解c(sp)-c(sp3)键和随后插入碳源来实现一个独特的方案。查阅现有公开文献后发现,将重氮衍生的金属碳烯插入内部炔的c(sp)-c(sp3)键仍然是未知的。
3.本发明人受铁催化的卡宾转移/插入反应的启发,设想利用经济和丰富的铁催化剂来介导c-c键的插入,以构建重要的炔取代季碳中心的新方法。在此,本发明人提出了一种新的铁催化在羧酸存在下选择性插入丙炔醇的c(sp)-c(sp3)键中,为形成炔取代季碳中心提供了方便的途径。这种转变始于丙炔醇与羧酸酯化原位生成酯基,避免了酮基的限制,然后在氯化铁和羧酸存在下将重氮金属-卡宾插入c(sp)-c(sp3)键中。
技术实现要素:
4.本发明目的在于克服现有技术的不足,提供一种高效、高选择性的铁催化重氮化合物选择插入炔丙醇的c(sp)
–
c(sp3)键构建季碳中心的方法,该方法以廉价易得的铁盐为促进剂,无需使用贵金属催化剂和配体,温和条件下高选择性的实现重氮插入构建新的季碳中心。本方法适用范围广泛,各种底物都能以较高收率得到目标产物。
5.为达上述目的,本发明采用的技术方案是:一种铁催化重氮化合物选择插入炔丙醇的c(sp)
–
c(sp3)键构建季碳中心的方法,该方法是在氩气气氛条件下,将式1所示的炔丙醇类化合物、式2所示的重氮化合物及式3所示的酸在铁催化剂存在下于溶剂乙酸乙酯中发生反应,制得式i所示的炔烃取代的季碳中心化合物,反应式如下:
[0006][0007]
其中,式1中,r1选自苯基、c
1-c6烷基、卤素苯基、甲苯基、丁基苯基或噻吩基;r2选自c
5-c
14
芳基、c
1-c
12
杂芳基、c
1-c6烷基、甲苯基、甲氧基苯基或丁基苯基;式2中,r3选自氢、苯基、卤素苯基、丁基苯基或硝基苯基;r4选自c
1-c
12
酯基;式3中,r5选自氢或c
1-c3烷基。
[0008]
较佳的,所述式1中,r1选自苯基、c
3-c6烷基、卤素苯基、甲苯基、丁基苯基或噻吩基;r2选自c
6-c
10
芳基、c
3-c6烷基、甲苯基、甲氧基苯基、丁基苯基或噻吩基。式2中,r4选自c
1-c
10
酯基。式3中r5选自氢或甲基。
[0009]
较佳的,所述铁催化剂选自氯化铁、氯化亚铁、溴化铁、三乙酰丙酮铁或硝酸铁,优选为氯化铁。所述反应温度为室温。反应初始时,各物质用量分别为:重氮化合物为炔丙醇类化合物的2.0当量,酸为炔丙醇类化合物的5-20当量,铁催化剂为炔丙醇类化合物的0.1-0.3当量,溶剂为2.0ml。
[0010]
本发明的有益效果是:提出了一种铁催化重氮化合物选择性插入炔丙醇的c(sp)-c(sp3)键构建炔烃取代的季碳中心的新方法,该方法以廉价易得的铁盐为促进剂,以高收率得到一系列的目标产物。该方法具有反应底物适应范围广泛、简单高效的优点,特别适合于工业化生产。
具体实施方式
[0011]
以下结合具体实施例,对本发明进行进一步详细的描述,但本发明并不局限于此。
[0012]
下述实施例中所述实验方法,如无特殊说明,均为常规方法;所述试剂和原料,如无特殊说明,均可以从商业途径获得和/或根据已知的方法制备获得。
[0013]
实施例1-10为反应条件优化实验。
[0014]
实施例1
[0015][0016]
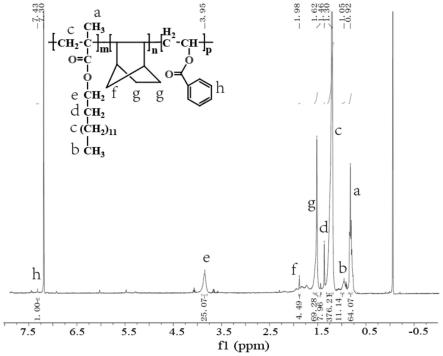
向schlenk瓶中加入式1a所示的炔丙醇类化合物(44.5mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)及氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时)。反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物(88%yield,d.r.=9:1),产物结构见式i-1,表征数据为:1hnmr(400mhz,cdcl3)δ:7.58-7.56(m,2h),7.48-7.45(m,2h),7.39-7.36(m,3h),7.27-7.25(m,3h),6.92-6.86(m,4h),6.50(s,1h),3.81(s,2.7h),3.70(s,0.3h),2.35(s,0.3h),2.22(s,2.7h),2.13(s,2.7h),2.06(s,0.3h);
13
c nmr(101mhz,cdcl3)δ:170.4,169.4,137.7,135.1,132.5,131.8(2),128.6,128.5,128.4,128.3,128.1,127.9,127.8,127.4,127.2,122.9,89.0,84.8,78.8,58.8,53.4,52.7,31.5,30.1,21.1(2);hrms m/z(esi)calcd for c
27h25
o4([m h]
)413.1747,found 413.1741。
[0017]
实施例2
[0018]
不加氯化铁,其余条件同实施例1,得到目标产物i-1的收率为0%。
[0019]
实施例3
nmr(500mhz,cdcl3)δ:7.46(d,j=8.0hz,2h),7.29(t,j=8.0hz,2h),7.09(t,j=7.5hz,1h),6.99(d,j=8.0hz,1h),6.72(d,j=2.5hz,1h),6.68-6.66(m,1h),3.75(s,3h),3.54(d,j=9.5hz,1h),3.42(d,j=9.5hz,1h),3.12(d,j=15.5hz,1h),2.69(t,j=7.5hz,1h),2.62-2.57(m,2h),1.27(s,3h),1.20(s,3h);
13
c nmr(125mhz,cdcl3)δ:178.1,158.4,139.4,137.5,128.8,128.5,128.4,124.5,120.2,113.2,112.1,59.5,55.3,49.2,41.4,39.2,38.2,23.9,21.0;hrms m/z(esi)calcd for c
21h24
no2([m h]
)322.1802,found 322.1808。
[0039]
实施例12
[0040][0041]
向schlenk瓶中加入式1a所示的炔丙醇类化合物(44.5mg,0.2mmol)、式2c所示对硝基苯基重氮乙酸乙酯(94.0mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物(78%yield,d.r.=1.5:1),产物结构式见式i-3,表征数据为:1h nmr(400mhz,cdcl3)δ:8.27-8.25(m,1h),8.18-8.10(m,1.2h),7.70-7.67(m,2h),7.58-7.53(m,1.8h),7.42-7.35(m,2h),7.28-7.18(m,3h),6.93-6.88(m,2h),6.52(s,0.4h),6.02(s,0.6h),4.32-4.22(m,2h),2.37(s,1.2h),2.24(s,3h),2.11(s,1.8h),1.25-1.16(m,3h);
13
c nmr(101mhz,cdcl3)δ:170.6,169.8,169.2,167.6,148.3,147.7,147.1,146.5,142.5,140.7,138.4,138.2,131.7,131.6,129.2,129.0,128.9,128.8,128.5,128.3,128.2(2),127.9,123.9,123.4,122.4,121.9,89.8,89.0,83.8,83.6,78.5,73.4,66.9,64.3,62.9,62.3,59.0,21.0,20.6,13.9(2);hrms m/z(esi)calcd for c
28h26
no6([m h]
)472.1755,found 472.1759。
[0042]
实施例13
[0043][0044]
向schlenk瓶中加入式1a所示的炔丙醇类化合物(44.5mg,0.2mmol)、式2d所示对氟苯基重氮乙酸甲酯(77.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=
10:1),得到目标产物i-4(82%yield,d.r.=2:1),产物结构表征数据为:1hnmr(500mhz,cdcl3)δ:7.58-7.56(m,1h),7.39-7.36(m,3h),7.28-7.17(m,5h),7.11-7.03(m,1h),6.96-6.91(m,3h),6.82(s,0.3h),6.48(s,0.7h),3.82(s,2h),3.70(s,1h),2.35(s,1h),2.23(s,2h),2.12(s,2h),2.08(s,1h);
13
c nmr(126mhz,cdcl3)δ:171.7,170.0,169.3,169.2,162.6(d,j
c-f
=245.0hz),161.8(d,j
c-f
=242.3hz),138.0,137.8,137.7,137.6,135.1,132.2,131.8(2),129.9(d,j
c-f
=8.1hz),128.8(d,j
c-f
=15.6hz),128.7,128.4,128.1,128.0,127.8,123.0(2),122.6,121.8,115.3(d,j
c-f
=20.9hz),115.0(d,j
c-f
=23.6hz),89.3,88.5,84.2,84.1,78.7,67.1,64.2,58.6,53.6,52.8,21.1(2);
19
f nmr(471mhz,cdcl3)d:-111.9(2);hrms m/z(esi)calcd for c
27h24
fo4([m h]
)431.1653,found 431.1647。
[0045]
实施例14
[0046][0047]
向schlenk瓶中加入式1a所示的炔丙醇类化合物(44.5mg,0.2mmol)、式2e所示对氯苯基重氮乙酸甲酯(84.0mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温下条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-5(83%yield,d.r.=1.1:1),表征数据为:1h nmr(400mhz,cdcl3)δ:7.57-7.55(m,1h),7.42-7.35(m,4.4h),7.29(t,j=2.0hz,1.6h),7.25-7.21(m,3h),7.17-7.14(m,1h),6.95-6.90(m,2h),6.83(s,0.4h),6.47(s,0.6h),3.81(s,1.6h),3.69(s,1.4h),2.35(s,1.4h),2.23(s,1.6h),2.12(s,1.6h),2.07(s,1.4h);
13
c nmr(101mhz,cdcl3)δ:171.8,170.1,169.3,169.2,137.9,137.8,137.4,135.2,134.4,133.7,133.5,132.2,131.7,129.0,128.9,128.8,128.7,128.6,128.5,128.3,128.1,128.0,127.9,127.2,122.6,121.8,89.3,88.5,84.4,84.1,78.6,73.6,67.1,63.9,60.3,58.3,53.5,52.8,21.1,21.0;hrms m/z(esi)calcd for c
27h24
clo4([m h]
)447.1358,found 447.1350。
[0048]
实施例15
[0049][0050]
向schlenk瓶中加入式1a所示的炔丙醇类化合物(44.5mg,0.2mmol)、式2f所示对溴苯基重氮乙酸甲酯(101.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气
气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-6(83%yield,d.r.=2.3:1),表征数据为:1h nmr(400mhz,cdcl3)δ:7.57-7.52(m,1.4h),7.46-7.44(m,0.6h),7.39-7.33(m,6h),7.23-7.15(m,2h),6.92(t,j=9.2hz,3h),6.82(s,0.3h),6.46(s,0.7h),3.81(s,2h),3.69(s,1h),2.35(s,0.9h),2.24(s,2.1h),2.12(s,2.1h),2.08(s,0.9h);
13
c nmr(101mhz,cdcl3)δ:171.8,170.0,169.3,169.2,138.0,137.8,135.2,134.3,132.2,132.0,131.7,131.5,130.2,129.3,129.2,128.9,128.8,128.7,128.4,128.1,128.0,127.9,122.6,121.8,89.4,84.3,78.6,73.7,67.0,64.0,58.4,53.5,52.8,21.1(2);hrms m/z(esi)calcd for c
27h24
bro4([m h]
)491.0852,found 491.0850。
[0051]
实施例16
[0052][0053]
向schlenk瓶中加入式1a所示的炔丙醇类化合物(44.5mg,0.2mmol)、式2g所示苯基重氮乙酸异丙酯(81.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-7(83%yield,d.r.>20:1),表征数据为:1h nmr(500mhz,cdcl3)δ:7.56-7.54(m,2h),7.51-7.49(m,2h),7.36(t,j=3.5hz,3h),7.25-7.24(m,3h),6.93-6.88(m,4h),6.51(s,1h),5.17-5.12(m,1h),2.22(s,3h),2.12(s,3h),1.33(d,j=6.5hz,3h),1.21(d,j=6.5hz,3h);
13
c nmr(126mhz,cdcl3)δ:169.4,169.1,137.6,135.3,132.7,131.7,128.3(3),128.2,127.9,127.8,127.5,123.2,88.9,85.2,78.8,70.0,59.2,21.5,21.3,21.1(2);hrms m/z(esi)calcd for c
29h29
o4([m h]
)441.2060,found 441.2068。
[0054]
实施例17
[0055][0056]
向schlenk瓶中加入式1a所示的炔丙醇类化合物(44.5mg,0.2mmol)、式2h所示苯基重氮乙酸苄基酯(100.8mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-8(83%yield,d.r.=4:1),表征数据为:1h nmr(500mhz,cdcl3)δ:
7.54-7.52(m,2h),7.48-7.46(m,2h),7.37-7.36(m,4h),7.32-7.29(m,4h),7.24-7.19(m,3h),6.92-6.87(m,4h),6.51(d,j=3.0hz,1h),5.36-5.32(m,1h),5.21-5.18(m,1h),2.35(s,0.6h),2.22(s,2.4h),1.99(s,2.4h),1.87(s,0.6h);
13
c nmr(126mhz,cdcl3)δ:171.3,169.5,169.4,137.7,135.5,135.3,134.9,132.5,131.8,131.7,128.4(2),128.3(3),128.0,127.9,127.8,127.7,127.5,123.0,89.1,84.9,78.8,67.4,67.1,64.4,59.0,29.6,21.1,20.9;hrms m/z(esi)calcd for c
33h29
o4([m h]
)489.2060,found 489.2064。
[0057]
实施例18
[0058][0059]
向schlenk瓶中加入式1a所示的炔丙醇类化合物(44.5mg,0.2mmol)、式2i所示苯基重氮乙酸丙烯酯(80.8mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)及氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-9(76%yield,d.r.=2:1),表征数据为:1h nmr(500mhz,cdcl3)δ:7.57-7.55(m,1.4h),7.50-7.48(m,1.6h),7.40-7.36(m,3h),7.33-7.31(m,1h),7.26-7.21(m,4h),7.15(d,j=8.0hz,0.3h),6.91(t,j=8.5hz,2.6h),6.88(s,0.4h),6.52(s,0.7h),5.93-5.85(m,0.7h),5.83-5.77(m,0.3h),5.36-5.32(m,0.7h),5.22-5.13(m,1.3h),4.79-4.75(m,0.7h),4.69-4.57(m,1.3h),2.35(s,1h),2.22(s,2h),2.11(s,2h),2.05(s,1h);
13
c nmr(126mhz,cdcl3)δ:171.3,169.4(2),137.7,137.4,135.0,132.5,131.8,131.7,131.4,128.6,128.4(2),128.3,128.0,127.9,127.8,127.5,127.4,123.0,122.0,118.6,118.0,89.1,84.8,78.8,66.4,66.1,64.4,58.9,21.1,21.0;hrms m/z(esi)calcd for c
29h27
o4([m h]
)439.1904,found 439.1900。
[0060]
实施例19
[0061][0062]
向schlenk瓶中加入式1b所示的炔丙醇类化合物(47.6mg,0.2mmol)、式2j所示重氮乙酸乙酯(45.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-10(79%yield,d.r.=1.5:1),表征数据为:1h nmr(500mhz,cdcl3)δ:7.42-7.40(m,1h),7.36-7.29(m,4h),7.24-7.22(m,2h),6.89-6.86(m,2h),6.15(d,j=
9.0hz,0.4h),6.08(d,j=10.5hz,0.6h),4.22-4.14(m,2h),4.05-4.00(m,1h),3.80(s,1.2h),3.79(s,1.8h),2.10(s,1.8h),1.92(s,1.2h),1.25-1.22(m,3h);
13
c nmr(126mhz,cdcl3)δ:170.6,170.3,169.3,159.5,159.4,131.9,131.7,130.0,128.7,128.6,128.2,128.1,126.1,125.9,122.0,114.0,113.9,87.0,86.3,84.8,84.4,65.7,64.5,61.2,55.9,55.3,55.2,20.8,20.6,14.1(2);hrms m/z(esi)calcd for c
22h23
o5([m h]
)367.1540,found 367.1548。
[0063]
实施例20
[0064][0065]
向schlenk瓶中加入式1c所示的炔丙醇类化合物(52.8mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-11(88%yield,d.r.》20:1),表征数据为:1h nmr(500mhz,cdcl3)δ:7.57-7.55(m,2h),7.45-7.43(m,2h),7.38(t,j=3.5hz,3h),7.26-7.24(m,3h),7.07(d,j=8.5hz,2h),6.93(d,j=8.5hz,2h),6.52(s,1h),3.81(s,3h),2.13(s,3h),1.21(s,9h);
13
c nmr(126mhz,cdcl3)δ:170.4,169.5,150.8,135.1,132.4,131.8,128.5,128.3(3),127.6,127.5,124.0,123.0,88.9,85.0,78.7,58.8,53.4,34.4,31.2,21.2;hrms m/z(esi)calcd for c
30h31
o4([m h]
)455.2217,found 455.2211。
[0066]
实施例21
[0067][0068]
向schlenk瓶中加入式1d所示的炔丙醇类化合物(41.6mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-12(83%yield,d.r.=1.1:1),表征数据为:1h nmr(500mhz,cdcl3)δ:7.57-7.55(m,1h),7.50-7.44(m,3h),7.40-7.37(m,3h),7.34-7.32(m,2h),7.26-7.13(m,4h),7.07(t,j=7.5hz,1h),7.00(d,j=7.5hz,1h),6.53(s,0.6h),5.93(s,0.4h),3.82(s,1.4h),3.72(s,1.6h),2.20(s,1.4h),2.14(s,1.6h);
13
c nmr(126mhz,cdcl3)δ:172.1,170.3,169.4,169.3,135.5,135.0,131.8,131.7,129.2,128.8,128.7,128.5,128.4(2),
128.3,128.1(2),128.0,127.8,127.7,127.6,127.5,127.4,127.3,127.1,122.8,122.0,89.1,84.7,78.9,74.4,58.7,53.4,52.6,21.1,20.7;hrms m/z(esi)calcd for c
26h23
o4([m h]
)399.1591,found 399.1595。
[0069]
实施例22
[0070][0071]
向schlenk瓶中加入式1e所示的炔丙醇类化合物(47.6mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-13(92%yield,d.r.=1.5:1),表征数据为:1h nmr(400mhz,cdcl3)δ:7.58-7.56(m,1h),7.47-7.44(m,1h),7.42-7.37(m,3h),7.33(s,1h),7.27-7.22(m,5h),6.96-6.94(m,1h),6.88-6.86(m,1h),6.84(s,0.4h),6.62-6.60(m,1h),6.49(s,0.6h),3.81(s,1.2h),3.81(s,1.8h),3.70(s,1.2h),3.70(s,1.8h),2.13(s,1.8h),2.06(s,1.2h);
13
c nmr(101mhz,cdcl3)δ:172.3,170.4,169.5,169.4,159.3,158.9,138.9,135.1,131.8,131.7,129.2,128.6,128.5,128.4,128.3,128.1,127.7,127.5,127.4,127.2,122.9,122.0,113.2,112.5,89.1,88.1,84.8,84.5,78.6,67.1,64.0,58.8,55.2,55.0,53.4,52.7,31.4,30.2,21.2,21.0;hrms m/z(esi)calcd for c
27h25
o5([m h]
)429.1697,found 429.1693。
[0072]
实施例23
[0073][0074]
向schlenk瓶中加入式1f所示的炔丙醇类化合物(50.4mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-14(84%yield,d.r.=9:1),表征数据为:1h nmr(400mhz,cdcl3)δ:7.75-7.72(m,1h),7.49-7.47(m,2h),7.38-7.36(m,2h),7.19-7.16(m,5h),7.13-7.11(m,1h),7.01(t,j=0.8hz,1h),6.88(t,j=7.6hz,1h),6.44(d,j=8.0hz,1h),3.83(s,2.7h),3.77(s,0.3h),3.72(s,0.3h),3.14(s,2.7h),2.39(s,2.7h),2.35(s,0.3h),2.10(s,
2.7h),1.94(s,0.3h);
13
c nmr(101mhz,cdcl3)δ:170.5,169.3,156.5,138.5,134.8,131.6(2),129.4,129.3,129.1(2),128.9,128.8,127.9,127.8,127.7,127.6,127.5,125.2,124.4,120.0,119.4,119.3,110.2,109.4,88.6,84.8,71.5,70.6,58.9,57.7,55.8,54.6,53.3,53.1,31.4,30.2,21.5,21.1;hrms m/z(esi)calcd for c
28h27
o5([m h]
)443.1853,found 443.1857。
[0075]
实施例24
[0076][0077]
向schlenk瓶中加入式1f所示的炔丙醇类化合物(50.4mg,0.2mmol)、式2j所示重氮乙酸乙酯(45.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-15(77%yield,d.r.=1:1),表征数据为:1h nmr(500mhz,cdcl3)δ:7.47-7.40(m,1h),7.29-7.27(m,2h),7.09-7.07(m,2h),7.01(s,1h),6.96-6.94(m,1h),6.89(d,j=8.5hz,1h),6.21(s,0.5h),6.19(s,0.5h),4.65(t,j=9.0hz,1h),4.20-4.13(m,2h),3.84(s,1.5h),3.81(s,1.5h),2.33(s,1.5h),2.29(s,1.5h),2.09(s,1.5h),2.05(s,1.5h),1.23-1.20(m,3h);
13
c nmr(126mhz,cdcl3)δ:170.7,170.3,169.5,169.3,157.5(2),138.7,138.5,131.8,131.6,129.7,129.6,129.2,129.1,128.9,128.8,123.2,122.9,120.6(2),119.2(2),110.9,110.8,86.4,86.2,84.5,84.0,65.1,64.1,61.0,60.3,55.7,48.8,48.4,21.4(2),20.9,20.6,14.2,14.1;hrms m/z(esi)calcd for c
23h25
o5([m h]
)381.1697,found 381.1691。
[0078]
实施例25
[0079][0080]
向schlenk瓶中加入式1g所示的炔丙醇类化合物(50.4mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-16(82%yield,d.r.=14:1),表征数据为:1h nmr(400mhz,cdcl3)δ:
7.75(d,j=7.6hz,1h),7.42-7.36(m,5h),7.19-7.14(m,5h),7.01(s,1h),6.90(t,j=7.2hz,1h),6.45(d,j=8.0hz,1h),3.84(s,2.8h),3.78(s,0.2h),3.73(s,0.2h),3.14(s,2.8h),2.38(s,2.8h),2.33(s,0.2h),2.10(s,2.8h),1.94(s,0.2h);
13
c nmr(101mhz,cdcl3)δ:170.5,169.3,156.5,138.0,137.8,134.7,132.3,129.3,129.2,129.1,128.8(2),128.2,128.1,127.8,127.7,127.6,127.5,125.1,124.3,122.8,119.4,119.3,110.2,109.4,88.6,85.2,71.5,70.6,58.8,57.7,55.8,54.6,53.3,53.1,31.4,30.2,21.2,21.1;hrms m/z(esi)calcd for c
28h27
o5([m h]
)443.1853,found 443.1857。
[0081]
实施例26
[0082][0083]
向schlenk瓶中加入式1g所示的炔丙醇类化合物(50.4mg,0.2mmol)、式2j所示重氮乙酸乙酯(45.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-17(76%yield,d.r.=1.5:1),表征数据为:1h nmr(400mhz,cdcl3)δ:7.47-7.40(m,1h),7.30-7.28(m,1h),7.22-7.14(m,2h),7.12-7.07(m,1h),7.02-6.95(m,2h),6.89(d,j=8.0hz,1h),6.21(d,j=2.4hz,0.4h),6.19(d,j=2.0hz,0.6h),4.65(t,j=8.0hz,1h),4.21-4.16(m,2h),3.84(s,1.8h),3.82(s,1.2h),2.30(s,1.8h),2.25(s,1.2h),2.09(s,1.2h),1.92(s,1.8h),1.25-1.20(m,3h);
13
c nmr(101mhz,cdcl3)δ:170.7,170.3,169.5,169.4,157.4(2),137.8,137.7,132.5,132.3,129.7,129.5,129.4,129.3,129.2,129.1,128.9,128.7,128.0,127.9,123.1,122.8,122.0(2),120.6(2),110.9,110.7,86.4,86.2,84.8,84.3,65.0,64.0,61.0,55.7,48.7,48.3,31.4,30.2,21.1(2),20.9,20.7,14.1;hrms m/z(esi)calcd for c
23h25
o5([m h]
)381.1697,found 381.1691。
[0084]
实施例27
[0085][0086]
向schlenk瓶中加入式1h所示的炔丙醇类化合物(51.7mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成
后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-18(67%yield,d.r.=1.1:1),表征数据为:1h nmr(500mhz,cdcl3)δ:7.86-7.79(m,2h),7.71(d,j=7.5hz,0.4h),7.60(d,j=8.0hz,0.6h),7.57-7.50(m,4h),7.47-7.45(m,1h),7.41-7.36(m,5h),7.25-7.18(m,3.5h),7.10-7.07(m,0.5h),7.02(s,0.4h),6.69(s,0.6h),3.85(s,1.6h),3.72(s,1.4h),2.18(s,1.6h),2.10(s,1.4h);
13
c nmr(126mhz,cdcl3)δ:172.0,170.3,169.5,138.6,135.9,135.0,133.1,133.0,132.8,132.6,132.4,131.8(2),128.7,128.6,128.5,128.4,128.3,128.1,127.6,127.5(2),127.4,127.3,126.5(2),126.3,126.2,126.0,125.9,125.8,125.7,122.8,121.9,89.3,84.7,79.0,58.8,53.5,52.8,21.2,21.0;hrms m/z(esi)calcd for c
30h25
o4([m h]
)449.1747,found 449.1753。
[0087]
实施例28
[0088][0089]
向schlenk瓶中加入式1i所示的炔丙醇类化合物(42.8mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-19(86%yield,d.r.=2:1),产物结构表征数据为:1h nmr(500mhz,cdcl3)δ:7.65-7.55(m,1h),7.51-7.49(m,0.4h),7.39-7.33(m,5h),7.27-7.21(m,5.6h),7.11-7.06(m,0.4h),7.02-6.99(m,0.6h),6.73(s,0.7h),6.62(s,0.3h),3.79(s,1h),3.77(s,2h),2.12(s,1h),2.05(s,2h);
13
c nmr(126mhz,cdcl3)d:171.3,170.1,169.3(2),140.9,138.8,137.7,131.8(2),129.3,128.8,128.7,128.6,128.4,128.3,128.1,128.0,127.9,127.5,127.2,126.0(2),125.8,125.6,125.5,121.9,89.3,88.1,84.6,84.3,75.3,67.6,62.6,53.5,53.0,21.0,20.9;hrms m/z(esi)calcd for c
24h21
o4s([m h]
)405.1155,found 405.1147。
[0090]
实施例29
[0091][0092]
向schlenk瓶中加入式1j所示的炔丙醇类化合物(34.8mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气
氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-20(60%yield,d.r.》20:1),表征数据为:1h nmr(500mhz,cdcl3)δ:7.47-7.46(m,2h),7.43-7.41(m,2h),7.39-7.38(m,3h),7.30-7.29(m,3h),5.93(s,1h),3.72(s,3h),2.20(s,3h),1.81-1.76(m,2h),1.58-1.53(m,2h),0.98(t,j=7.5hz,3h);
13
c nmr(126mhz,cdcl3)δ:170.3,169.3,133.7,131.6,129.2,128.7,128.2(2),127.6,122.6,90.2,84.6,74.4,62.6,52.6,39.9,20.6,18.5,13.7;hrms m/z(esi)calcd for c
23h25
o4([m h]
)365.1747,found 365.1743。
[0093]
实施例30
[0094][0095]
向schlenk瓶中加入式1k所示的炔丙醇类化合物(50.4mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-21(87%yield,d.r.=2:1),产物结构表征数据为:1h nmr(400mhz,cdcl3)δ:7.47-7.44(m,3h),7.40-7.31(m,2h),7.26-7.24(m,2.3h),7.18(d,j=8.0hz,1.3h),7.12(d,j=8.0hz,0.7h),7.03(d,j=8.0hz,0.7h),6.96-6.94(m,1h),6.88-6.84(m,1h),6.61-6.60(m,1h),6.59(s,0.3h),6.48(s,0.7h),3.80(s,3h),3.69(s,1h),3.68(s,2h),2.38(s,2h),2.29(s,1h),2.12(s,2h),2.05(s,1h);
13
c nmr(101mhz,cdcl3)δ:172.2,170.4,169.4,159.2,158.8,139.0,138.8,138.6,135.2,131.6(2),129.2,129.0,128.8,128.4,128.2,127.7,127.3,127.2,119.7,118.9,113.1,112.5,89.1,88.2,84.1,83.8,78.6,67.1,64.0,58.7,55.1,55.0,53.3,52.6,21.5,21.4,21.1,20.9;hrms m/z(esi)calcd for c
28h27
o5([m h]
)443.1853,found 443.1857。
[0096]
实施例31
[0097][0098]
向schlenk瓶中加入式1k所示的炔丙醇类化合物(50.4mg,0.2mmol)、式2j所示重氮乙酸乙酯(45.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=
10:1),得到目标产物i-22(81%yield,d.r.=1:1),产物结构表征数据为:1h nmr(500mhz,cdcl3)δ:7.35-7.32(m,2h),7.31-7.29(m,1h),7.14-7.09(m,2h),7.04-7.02(m,1h),6.88-6.86(m,2h),6.14(d,j=9.0hz,0.5h),6.07(d,j=10.5hz,0.5h),4.21-4.13(m,2h),4.04-3.99(m,1h),3.80(s,1.5h),3.79(s,1.5h),2.34(s,1.5h),2.30(s,1.5h),2.10(s,1.5h),1.92(s,1.5h),1.25-1.21(m,3h);
13
c nmr(126mhz,cdcl3)δ:170.7,170.3,169.4,159.5,159.3,138.9,138.8,131.8,131.6,130.0,129.0,128.8,126.1,125.9,118.9,114.0,113.8,87.2,86.5,84.1,83.7,65.8,64.6,61.2(2),55.9,55.3,55.2,21.5,21.4,20.9,20.7,14.1(2);hrms m/z(esi)calcd for c
23h25
o5([m h]
)381.1697,found 381.1691。
[0099]
实施例32
[0100][0101]
向schlenk瓶中加入式1l所示的炔丙醇类化合物(58.8mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-23(82%yield,d.r.=4:1),产物结构表征数据为:1h nmr(500mhz,cdcl3)δ:7.50-7.41(m,4h),7.34(d,j=2.0hz,1h),7.20(d,j=8.0hz,2h),7.14(d,j=8.0hz,0.5h),7.04(d,j=8.0hz,0.5h),6.96(d,j=9.0hz,2h),6.88-6.84(m,1h),6.61(d,j=9.0hz,2h),6.49(s,1h),3.82(s,0.6h),3.81(s,2.4h),3.70(s,3h),2.65(t,j=8.0hz,2h),2.13(s,2.4h),2.06(s,0.6h),1.64-1.60(m,2h),1.38(t,j=7.5hz,2h),0.95(t,j=7.5hz,2.4h),0.90(t,j=7.5hz,0.6h);
13
c nmr(126mhz,cdcl3)δ:172.3,170.4,169.4,159.2,158.9,143.8,143.6,135.3,131.7,131.6,129.2,128.4(2),128.2(2),127.7,127.4,120.0,119.1,113.2,112.5,89.2,84.0,78.6,58.8,55.2,55.0,53.4,52.7,35.6,35.5,33.4,33.3,31.5,30.1,22.3,21.1,13.9;hrms m/z(esi)calcd for c
31h33
o5([m h]
)485.2323,found 485.2317。
[0102]
实施例33
[0103][0104]
向schlenk瓶中加入式1l所示的炔丙醇类化合物(58.8mg,0.2mmol)、式2j所示重氮乙酸乙酯(45.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成
后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-24(71%yield,d.r.=1.1:1),产物结构表征数据为:1h nmr(500mhz,cdcl3)δ:7.35-7.31(m,3h),7.15-7.09(m,2h),7.04(d,j=8.0hz,1h),6.88-6.86(m,2h),6.14(d,j=9.0hz,0.5h),6.07(d,j=10.5hz,0.5h),4.21-4.12(m,2h),4.04-3.99(m,1h),3.80(s,1.4h),3.79(s,1.6h),2.60-2.54(m,2h),2.10(s,1.6h),1.91(s,1.4h),1.59-1.52(m,2h),1.34-1.31(m,2h),1.25-1.21(m,3h),0.93-0.88(m,3h);
13
c nmr(126mhz,cdcl3)δ:170.7,170.3,169.4,159.5,159.4,143.9,143.8,131.8,131.6,130.0,128.3,128.2,126.1,126.0,119.1,114.0,113.9,87.2,86.5,84.1,83.7,65.8,64.6,61.2(2),55.9,55.3,55.2,35.6,35.5,33.3,29.7,22.2(2),20.9,20.7,14.1(2),13.9(2);hrms m/z(esi)calcd for c
26h31
o5([m h]
)423.2166,found 423.2160。
[0105]
实施例34
[0106][0107]
向schlenk瓶中加入式1m所示的炔丙醇类化合物(54.4mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-25(91%yield,d.r.=1:1),产物结构表征数据为:1h nmr(500mhz,cdcl3)δ:7.50-7.47(m,1h),7.44-7.43(m,1h),7.40-7.32(m,4h),7.27-7.24(m,2h),7.21-7.18(m,1h),7.14-7.12(m,1h),6.93-6.87(m,2h),6.83(s,0.5h),6.61-6.58(m,1h),6.49(s,0.5h),3.81(s,3h),3.69(s,3h),2.12(s,1.5h),2.05(s,1.5h);
13
c nmr(126mhz,cdcl3)δ:172.1,170.1,169.4,169.3,159.3,158.9,138.9,134.9,134.7,134.6,133.0(2),130.6,130.4,129.1,128.7,128.5(2),128.4(2),127.6,127.5,127.3,127.2,121.3,120.4,113.2,112.5,88.0,86.9,86.0,85.5,78.5,67.0,64.0,58.9,55.2,55.0,53.4,52.7,21.1,20.9;hrms m/z(esi)calcd for c
27h24
clo5([m h]
)463.1307,found 463.1301。
[0108]
实施例35
[0109][0110]
向schlenk瓶中加入式1m所示的炔丙醇类化合物(54.4mg,0.2mmol)、式2j所示重氮乙酸乙酯(45.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成
后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-26(83%yield,d.r.=1.5:1),产物结构表征数据为:1h nmr(400mhz,cdcl3)δ:7.34-7.31(m,3h),7.28-7.26(m,1.8h),7.22-7.14(m,1.2h),6.89-6.86(m,2h),6.12(d,j=8.8hz,0.6h),6.06(d,j=10.4hz,0.4h),4.24-4.13(m,2h),4.04-3.98(m,1h),3.80(s,3h),2.11(s,1.2h),1.93(s,1.8h),1.23(t,j=7.2hz,3h);
13
c nmr(101mhz,cdcl3)δ:170.5,170.2,169.4,169.3,159.6,159.4,134.9,134.7,133.1,132.9,129.9,128.6,128.5,125.9,125.8,120.4(2),114.1,113.9,85.9,85.8,85.4,85.2,65.6,64.4,61.3,55.7,55.2(2),20.8,20.6,14.1(2);hrms m/z(esi)calcd for c
22h22
clo5([m h]
)401.1150,found 401.1158。
[0111]
实施例36
[0112][0113]
向schlenk瓶中加入式1n所示的炔丙醇类化合物(63.2mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-27(89%yield,d.r.=2:1),产物结构表征数据为:1h nmr(400mhz,cdcl3)δ:7.58-7.50(m,1h),7.43-7.40(m,2h),7.38-7.36(m,1h),7.35-7.32(m,3h),7.26-7.21(m,3h),7.06(d,j=8.4hz,0.4h),6.95(d,j=8.4hz,0.6h),6.88-6.82(m,2h),6.62-6.59(m,0.7h),6.49(d,j=2.0hz,0.3h),3.81(s,3h),3.70(s,2h),3.69(s,1h),2.12(s,1h),2.05(s,2h);
13
c nmr(101mhz,cdcl3)δ:172.2,172.1,169.4,169.3,159.3,158.9,139.0,133.2,131.8,131.7,131.6,131.4,130.6,129.2,129.1,128.6,128.5,128.4,128.3,128.1,127.4(2),127.3,127.2,122.0,120.9,113.2,112.5,89.0,88.1,84.9,84.5,78.6,78.5,67.1,64.0,55.2,55.0,53.4(2),52.7,52.6,21.1,20.9;hrms m/z(esi)calcd for c
27h24
bro5([m h]
)507.0802,found 507.0806。
[0114]
实施例37
[0115][0116]
向schlenk瓶中加入式1n所示的炔丙醇类化合物(63.2mg,0.2mmol)、式2j所示重氮乙酸乙酯(45.6mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成
后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-28(82%yield,d.r.=1.1:1),产物结构表征数据为:1h nmr(400mhz,cdcl3)δ:7.42(t,j=9.2hz,1h),7.38-7.29(m,3h),7.27-7.23(m,2h),6.89-6.86(m,2h),6.16-6.04(m,1h),4.22-4.15(m,2h),4.05-3.98(m,1h),3.80(s,3h),2.11(s,1.4h),1.93(s,1.6h),1.27-1.22(m,3h);
13
c nmr(101mhz,cdcl3)δ:170.5,170.3,169.4,169.3,159.5,159.4,133.3,133.1,131.9,131.7,131.5,131.4,129.9(2),128.7,128.6,128.2,128.1,114.0,113.9,86.3,86.0,84.8,84.4,65.7,65.6,64.5,64.4,61.2(2),55.8,55.2,20.8,20.6,14.1(2);hrms m/z(esi)calcd for c
22h22
bro5([m h]
)445.0645,found 445.0651。
[0117]
实施例38
[0118][0119]
向schlenk瓶中加入式1o所示的炔丙醇类化合物(48.8mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)及氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-29(82%yield,d.r.=5:1),产物结构表征数据为:1h nmr(400mhz,cdcl3)δ:7.43-7.41(m,2h),7.33-7.32(m,2h),7.30-7.29(m,1h),7.25-7.24(m,2h),7.03-7.01(m,1h),6.94-6.90(m,2h),6.61(d,j=8.4hz,2h),6.48(s,1h),3.79(s,3h),3.73(s,0.5h),3.68(s,2.5h),2.12(s,2.5h),2.04(s,0.5h);
13
c nmr(101mhz,cdcl3)δ:172.1,170.0,169.4,169.3,159.2,134.8,132.7,132.1,129.4,129.0,128.8,128.5,128.4,128.3(2),128.2,127.4,127.2(2),126.9,126.7,126.5,122.6,121.7,113.2,112.5,88.8,88.5,82.4,81.6,78.6,59.0,55.1,54.9,53.4,52.7,21.1,20.8;hrms m/z(esi)calcd for c
25h23
o5s([m h]
)435.1261,found 435.1265。
[0120]
实施例39
[0121][0122]
向schlenk瓶中加入式1p所示的炔丙醇类化合物(43.6mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)及氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=
10:1),得到目标产物i-30(67%yield,d.r.=4:1),产物结构表征数据为:1h nmr(500mhz,cdcl3)δ:7.37-7.35(m,2h),7.31-7.27(m,4h),7.23-7.18(m,1h),6.87-6.84(m,2h),6.61(d,j=3.5hz,0.8h),6.58(d,j=8.5hz,0.2h),3.82(s,2.4h),3.76(s,0.6h),3.70(s,0.6h),3.67(s,2.4h),2.11(s,0.6h),2.03(s,2.4h),1.35(s,1.8h),1.04(s,7.2h);
13
c nmr(126mhz,cdcl3)δ:172.4,170.8,169.4,158.8,158.6,139.2,139.1,130.8(2),130.5,129.3,128.2,128.0,127.3(2),127.2,127.1,112.9,112.3,97.6,97.0,78.3,74.1,73.7,66.8,64.0,55.2,55.0,53.1,52.5,30.9,30.8,30.3,27.8,27.3,21.1,21.0;hrms m/z(esi)calcd for c
25h29
o5([m h]
)409.2010,found 409.2014。
[0123]
实施例40
[0124][0125]
向schlenk瓶中加入式1r所示的炔丙醇类化合物(43.6mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)及氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-31(76%yield,d.r.=6.5:1),产物结构表征数据为:1h nmr(500mhz,cdcl3)δ:7.36-7.34(m,2h),7.30-7.27(m,4h),7.23-7.17(m,1h),6.87-6.84(m,2h),6.61(t,j=2.0hz,1h),3.82(s,2.6h),3.76(s,0.4h),3.69(s,0.4h),3.67(s,2.6h),2.06-2.04(m,2h),2.02(s,3h),1.32-1.27(m,2h),1.22-1.17(m,2h),0.97(t,j=7.0hz,0.4h),0.80(t,j=7.5hz,2.6h);
13
c nmr(126mhz,cdcl3)δ:172.4,170.8,169.5,159.1,158.8,139.1,130.8,130.7,130.4,129.2,128.3,128.1,127.3(2),127.1,126.9,113.0,112.4,89.4,78.4,75.3,67.0,63.9,55.2,55.0,53.2,52.5,30.6,30.1,22.0,21.7,21.1,20.9,18.8,18.3,13.6,13.5;hrms m/z(esi)calcd for c
25h29
o5([m h]
)409.2010,found 409.2016。
[0126]
实施例41
[0127][0128]
向schlenk瓶中加入式1s所示的炔丙醇类化合物(32.2mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3a所示乙酸(ch3cooh,120.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-32(75%yield,d.r.=4:1),产物结构表征数据为:1h nmr(400mhz,
cdcl3)δ:7.40-7.36(m,1h),7.28(d,j=8.8hz,2h),7.22-7.19(m,3h),7.15-7.10(m,1h),6.81-6.74(m,2h),6.54(s,0.7h),6.50(d,j=8.8hz,0.3h),3.73(s,2.4h),3.67(s,0.6h),3.63(s,0.6h),3.58(s,2.4h),2.33(t,j=7.2hz,0.4h),2.02-1.96(m,1.6h),1.93(s,3h),1.25-1.19(m,4h),1.14-1.09(m,4h),0.83(t,j=6.8hz,0.6h),0.77(t,j=7.2hz,2.4h);
13
c nmr(101mhz,cdcl3)δ:172.3,170.7,169.4,169.3,159.0,158.7,138.9,135.5,130.7,130.6,130.4,129.1,128.7,128.2,127.5,127.3,127.2,127.0,112.9,112.3,89.7,89.4,86.5,84.7,78.3,75.2,74.3,66.8,63.8,58.1,55.0,54.9,52.5,31.3,31.2,28.5,28.4,28.2,27.9,22.5,22.4,21.0,20.9,19.0,18.6,13.9;hrms m/z(esi)calcd for c
27h33
o5([m h]
)437.2323,found 437.2327。
[0129]
实施例42
[0130][0131]
向schlenk瓶中加入式1a所示的炔丙醇类化合物(44.4mg,0.2mmol)、式2a所示苯基重氮乙酸甲酯(70.4mg,0.4mmol)、式3b所示甲酸(hcooh,92.1mg,2.0mmol)和氯化铁(fecl3,6.5mg,0.04mmol),再加入溶剂乙酸乙酯(etoac,2.0ml),然后将反应器在氩气气氛、室温条件下搅拌反应,经tlc监测反应进程至原料消失(反应时间为8小时),反应完成后,将反应液减压浓缩除去溶剂,将残余物经柱层析分离(洗脱溶剂为:乙酸乙酯/正已烷=10:1),得到目标产物i-33(82%yield,d.r.=2:1),产物结构表征数据为:1h nmr(500mhz,cdcl3)δ:8.08(s,0.7h),8.01(s,0.3h),7.51-7.50(m,1.5h),7.39-7.37(m,1.5h),7.32-7.29(m,3h),7.27-7.25(m,1h),7.20-7.18(m,2h),7.15-7.13(m,2h),6.92-6.91(m,0.3h),6.86-6.81(m,3h),6.53(s,0.7h),3.74(s,2h),3.63(s,1h),2.28(s,1h),2.15(s,2h);
13
c nmr(126mhz,cdcl3)δ:172.0,170.3,159.5,159.4,138.4,138.0,137.6,134.7,131.7,128.8,128.7,128.6,128.5(2),128.3,128.1,127.9(2),127.6,127.3(2),122.7,121.8,89.3,88.8,84.3,83.8,78.6,66.7,64.2,58.6,53.5,52.8,21.1,21.0;hrms m/z(esi)calcd for c
26h23
o4([m h]
)399.1591,found 399.1597。
[0132]
实施例43控制实验
[0133]
[0134]
本技术人进行了一些机理探究的实验。当将2,2,6,6,6-四甲基哌啶亚氧基(tempo)或丁基羟基甲苯(bht)引入反应体系时,单碳插入过程顺利,说明自由基途径可能没有参与该反应。
[0135]
由此可知,本发明的可能反应机理推导如下式所示:
[0136][0137]
以上所述实施例仅为本发明的优选实施例,而并非本发明可行实施的穷举。对于本领域技术人员而言,在不背离本发明原理和精神的前提下,对其所作出的任何显而易见的改动,都应当被认为包含在本发明的权利要求保护范围之内。
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。