1.本发明应用于有机合成技术领域,是一种亚砜亚胺基二氢吡喃酮类衍生物的制备方法。
背景技术:
2.亚砜亚胺类化合物是砜基的一种含氮类似物,由于其独特的结构与性质,自从被发现以来一直备受关注,并展示出广泛的生物活性(eur.j.med.chem.2017,126,225
–
245)。此外,亚砜亚胺也被用作试剂(acc.chem.res.1973,6,341
–
347)、手性助剂(j.am.chem.soc.1995,117,2453
–
2466)、手性配体(j.am.chem.soc.2001,123,3830
–
3831)、有机催化剂(j.org.chem.2012,8,1443
–
1451)等。通过亚砜亚胺能够实现各种转化反应,包括芳基化、烷基化、乙烯基化、炔基化和其他反应(asian j.org.chem.2020,9,2035-2082及chem.rec.2021,21,396-416)。但是,目前还没有合成亚砜亚胺基二氢吡喃酮的方法被报道出来。
3.含有n-糖苷键结构的氮苷糖具有广泛的药理作用,是一些抗生素、抗肿瘤药物和抗病毒药物等的重要母环结构(chem.rev.1996,96,683及synlett.2009,07,1154-1156及synthesis.2009,4143)。使用achmatowicz重排反应的产物二氢吡喃酮为糖基供体的“de novo”从头合成糖的策略已成为合成糖苷化合物的重要方法之一(chem.asian j.2017,12,1027-1042)。2006年,o’doherty等人以6-氯嘌呤和boc保护的二氢吡喃酮为原料,通过形成π-烯丙基钯中间体,实现了高对映选择性及非对映选择性合成氮苷糖类衍生物(org.lett.2006,8,293
–
296)。2020年,申请人以boc保护的二氢吡喃酮为供体,甲基香豆素为受体,通过钯催化烯丙基-烯丙基偶联反应,在温和条件下实现了高对映选择性及非对映选择性合成碳苷糖类衍生物(adv.synth.catal.2021,363,846-850)。除了钯催化剂以外,铱也能够催化类似的反应。2018年,申请人在温和的条件下,以羟基无保护的二氢吡喃酮为供体,通过形成π-烯丙基铱中间体实现高区域选择性和非对映选择性合成氮杂糖类衍生物(j.org.chem.2018,83,12822-12830)。但是,上述方法多是使用了昂贵的金属催化剂来用于催化。因此,发展一种无金属催化的de novo合成糖苷类化合物的新策略是非常重要的,尤其是对金属残留指标要求较高的制药行业中。
技术实现要素:
4.本发明的目的是克服现有技术中的不足,本发明提出了一种亚砜亚胺基二氢吡喃酮类衍生物的制备方法,该方法条件温和、操作简单、官能团兼容性好,避免了传统方法中使用的贵金属催化剂,包括以亚砜亚胺和二氢吡喃酮为原料的制备亚砜亚胺基二氢吡喃酮类衍生物的方法。
5.本发明是通过如下技术方案实现上述目的的,一种亚砜亚胺基二氢吡喃酮类衍生物的制备方法,
6.在有机溶剂中,在(pho)2p(o)oh催化作用下,式i所示亚砜亚胺化合物与式ii所示
二氢吡喃酮化合物的发生亲核取代反应制备式iii所示亚砜亚胺基二氢吡喃酮类衍生物,反应式如下:
[0007][0008]
其中,r1和r2独立地为烷基或芳基,r3为氢、烷基或芳基;i为亚砜亚胺类化合物,ⅱ为二氢吡喃酮类化合物;
[0009]
优选地,r1、r2和r3中烷基为c1-c6的烷基、c3-c8的环烷基。
[0010]
优选地,所述r1、r2和r3中芳基为苯基或任选位置被ra取代的苯基;所述ra为c1-c6的烷基、c1-c6的烷氧基、卤素;
[0011]
优选地,反应温度为0-60℃,优选为20-30℃,反应时间为12-72h,制备得到收率不低于44%的亚砜亚胺基二氢吡喃酮类衍生物。
[0012]
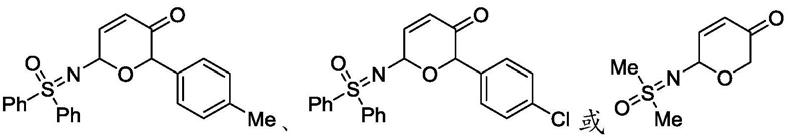
优选地,所述式iii所示亚砜亚胺基二氢吡喃酮类衍生物为
[0013]
中任意一种。
[0014]
优选地,式i所示亚砜亚胺化合物与式ii所示二氢吡喃酮化合物的摩尔比为1:1-10:1,优选为2:1。
[0015]
优选地,所述的(pho)2p(o)oh的用量为二氢吡喃酮的20mol%。
[0016]
优选地,所述的有机溶剂为乙腈、甲苯、氯仿、二氯甲烷、1,2-二氯乙烷、四氢呋喃、丙酮中的一种或两种以上混合,优选溶剂为氯仿,或二氯甲烷。
[0017]
本发明的有益效果:
[0018]
1)本发明中亚砜亚胺和二氢吡喃酮类化合物的布朗斯特酸催化偶联制备方法反应条件温和,产物收率不低于44%
[0019]
2)本发明方法的原料简单易得,底物范围广,条件温和,反应易操作,产率中等到良好。
[0020]
3)本发明方法避免了传统上使用的贵金属钯、铱等催化剂,进一步丰富了“de novo”从头合成糖苷类化合物的策略,可为设计与合成氮苷糖类化合物提供方法学基础和指导,制备得到的亚砜亚胺基二氢吡喃酮类衍生物具有潜在的生理活性。
具体实施方式
[0021]
实施例1:
[0022]
6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0023]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二苯基亚砜亚胺(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到无色液体产物43mg,产率68%。
[0024][0025]1h nmr(400mhz,cdcl3)δ8.00(dd,j=9.7,7.8hz,4h),7.58
–
7.48(m,6h),7.00(dd,j=10.2,3.3hz,1h),6.09(d,j=10.3hz,1h),5.46(d,j=2.8hz,1h),4.69(d,j=16.6hz,1h),4.05(d,j=16.6hz,1h).
13
c nmr(101mhz,cdcl3)δ195.40,150.50,140.34,139.97,133.06,133.03,129.38,129.33,128.62,128.18,125.80,78.35,67.52.高分辨质谱(esi,m/z):[m h]
计算值:c
17h16
nso3,314.0845;测量值:314.0849.
[0026]
实施例2:
[0027]
2-甲基-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0028]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于二氯乙烷(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-甲基-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到无色液体产物42mg,产率64%,dr=2:1。
[0029][0030]
主要异构体:1h nmr(600mhz,cdcl3)δ8.00
–
7.97(m,4h),7.52
–
7.47(m,6h),6.93(dd,j=10.1,3.7hz,1h),6.01(d,j=10.1hz,1h),5.51(d,j=3.1hz,1h),4.83(q,j=6.7hz,1h),1.24(d,j=6.8hz,3h).
[0031]
次要异构体:1h nmr(600mhz,cdcl3)δ8.07
–
8.02(m,4h),7.57
–
7.52(m,6h),7.04(dd,j=10.1,1.2hz,1h),6.08(dd,j=10.1,1.7hz,1h),5.54(d,j=1.3hz,1h),4.04
–
3.99(m,1h),1.31(d,j=6.6hz,3h).
[0032]
13
c nmr(151mhz,cdcl3)δ197.89,196.85,152.65,149.17,140.86,140.09,132.96,132.91,132.89,129.31,129.26,129.21,129.07,128.89,128.49,128.10,126.94,125.04,124.79,80.65,77.58,75.92,70.65,15.65,15.14.高分辨质谱(esi,m/z):[m h]
计算值:328.1001;测量值:测量值:328.0999.
13
c nmr和高分辨质谱是针对两种异构体混合物的表征结果,其余实施例相同(针对存在dr值的实施例)。实施例3:
[0033]
2-乙基-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0034]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于二氯甲烷(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-乙基-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物48mg,产率70%,dr=2:1。
[0035][0036]
主要异构体:1h nmr(600mhz,cdcl3)δ8.08
–
7.96(m,4h),7.56
–
7.45(m,6h),6.94(dd,j=10.1,3.6hz,1h),6.01(d,j=10.1hz,1h),5.51(d,j=2.9hz,1h),4.64(dd,j=8.0,3.9hz,1h),1.92
–
1.59(m,2h),0.90(t,j=7.4hz,3h).
[0037]
次要异构体:1h nmr(600mhz,cdcl3)δ8.08
–
7.96(m,4h),7.56
–
7.45(m,6h),7.05(dd,j=10.1,1.2hz,1h),6.08(dd,j=10.1,1.8hz,1h),5.56(d,j=1.3hz,1h),3.78
–
3.76(m,1h),1.92
–
1.59(m,2h),0.78(t,j=7.4hz,3h).
[0038]
13
c nmr(151mhz,cdcl3)δ197.58,196.46,152.75,149.36,140.65,140.10,132.93,132.85,129.31,129.27,129.18,129.07,128.93,128.69,128.11,127.96,127.38,125.43,80.83,80.70,77.30,75.73,22.98,22.89,9.62.高分辨质谱(esi,m/z):[m h]
理论值:342.1158;测量值:342.1162.
[0039]
实施例4:
[0040]
2-丙基-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0041]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-丙基-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物45mg,产率63%,dr=1.5:1。
[0042][0043]
主要异构体:1h nmr(400mhz,cdcl3)δ8.07
–
7.96(m,4h),7.57
–
7.43(m,6h),6.93(dd,j=10.1,3.6hz,1h),6.01(d,j=10.1hz,1h),5.49(d,j=3.6hz,1h),4.70(dd,j=8.7,3.7hz,1h),1.89
–
1.52(m,2h),1.43
–
1.12(m,2h),0.91(t,j=7.4hz,3h).
[0044]
次要异构体:1h nmr(400mhz,cdcl3)δ8.07
–
7.96(m,4h),7.57
–
7.43(m,6h),7.05(d,j=10.2hz,1h),6.08(dd,j=10.1,1.8hz,1h),5.55(s,1h),3.83(dd,j=9.1,2.0hz,1h),1.89
–
1.52(m,2h),1.43
–
1.12(m,2h),0.78(t,j=7.4hz,3h).
[0045]
13
c nmr(151mhz,cdcl3)δ197.71,196.67,152.71,149.33,141.07,140.55,140.05,132.93,132.85,132.78,129.32,129.27,129.17,129.05,128.92,128.72,128.14,127.97,127.36,125.37,124.79,80.66,79.26,77.30,74.56,31.62,31.59,18.46,18.19,13.93,13.67.高分辨质谱(esi,m/z):[m h]
理论值:356.1314;测量值:测量值:356.1319.实施例5:
[0046]
2-异丙基-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0047]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于thf(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-异丙基-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物48mg,产率68%,dr=2:1。
[0048][0049]
主要异构体:1h nmr(600mhz,cdcl3)δ8.07
–
7.95(m,4h),7.55
–
7.45(m,6h),6.94(dd,j=10.1,3.7hz,1h),6.01(d,j=10.0hz,1h),5.52(d,j=3.1hz,1h),4.56(d,j=3.4hz,1h),2.35(dddd,j=16.5,9.6,8.5,4.8hz,1h),1.00
–
0.63(m,6h).
[0050]
次要异构体:1h nmr(600mhz,cdcl3)δ8.07
–
7.95(m,4h),7.55
–
7.45(m,6h),7.11
–
7.05(m,1h),6.08(dd,j=10.1,1.6hz,1h),5.61(d,j=1.0hz,1h),3.68(s,1h),2.35
(dddd,j=16.5,9.6,8.5,4.8hz,1h),1.00
–
0.63(m,6h).
[0051]
13
c nmr(151mhz,cdcl3)δ197.69,196.55,152.87,149.29,141.86,141.06,140.62,140.20,132.93,132.90,132.75,132.68,129.29,129.27,129.14,129.03,128.87,128.81,128.10,127.80,125.83,83.83,80.93,78.89,77.19,28.51,28.09,19.09,16.54,16.08.高分辨质谱(esi,m/z):[m h]
理论值:356.1314;测量值:测量值:356.1317.
[0052]
实施例6:
[0053]
2-环丙基-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0054]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-环丙基-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物47mg,产率66%,dr=2:1。
[0055][0056]
主要异构体:1h nmr(400mhz,cdcl3)δ8.06
–
7.91(m,4h),7.58
–
7.42(m,6h),6.93(dd,j=10.1,3.5hz,1h),6.02(dd,j=10.1,1.0hz,1h),5.53
–
5.50(m,1h),4.14(d,j=7.6hz,1h),1.33
–
1.07(m,1h),0.64
–
0.10(m,4h).
[0057]
次要异构体:1h nmr(400mhz,cdcl3)δ8.06
–
7.91(m,4h),7.58
–
7.42(m,6h),7.02(dd,j=10.1,1.5hz,1h),6.09(dd,j=10.1,2.0hz,1h),5.53
–
5.50(m,1h),4.14(d,j=7.6hz,1h),1.33
–
1.07(m,1h),0.64
–
0.10(m,4h).
[0058]
13
c nmr(151mhz,cdcl3)δ195.82,195.04,151.32,148.25,139.95,139.50,139.43,138.69,131.91,131.84,131.78,128.26,128.19,128.09,128.01,127.86,127.57,127.06,126.84,126.81,126.21,124.46,80.71,79.46,76.73,76.69,10.07,9.82,1.99,1.46,0.03,-0.01.高分辨质谱(esi,m/z):[m h]
理论值:354.1158;测量值:测量值:354.1161.实施例7:
[0059]
2-环戊基-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0060]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-环戊基-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物45mg,产率59%,dr=1.5:1。
[0061][0062]
主要异构体:1h nmr(400mhz,cdcl3)δ8.06
–
7.95(m,4h),7.56
–
7.46(m,6h),6.92(dd,j=10.1,3.5hz,1h),6.00(dd,j=10.1,1.0hz,1h),5.51(dd,j=3.5,0.9hz,1h),4.61(d,j=5.5hz,1h),2.54
–
2.38(m,1h),1.74
–
1.17(m,8h).
[0063]
次要异构体:1h nmr(400mhz,cdcl3)δ8.06
–
7.95(m,4h),7.56
–
7.46(m,6h),7.05(dd,j=10.1,1.3hz,1h),6.07(dd,j=10.1,2.0hz,1h),5.61(dd,j=3.3,1.6hz,1h),3.81(dd,j=4.7,1.5hz,1h),2.54
–
2.38(m,1h),1.74
–
1.17(m,8h).
[0064]
13
c nmr(151mhz,cdcl3)δ197.72,196.70,152.55,149.20,141.64,140.91,
140.52,140.06,132.94,132.90,132.76,132.65,129.30,129.27,129.14,129.04,128.86,128.81,128.14,127.81,125.78,124.79,81.84,80.96,77.48,77.39,39.32,39.27,28.71,28.55,27.23,26.68,25.83,25.67,25.53,25.50.高分辨质谱(esi,m/z):[m h]
理论值:382.1471;测量值:382.1473.
[0065]
实施例8:
[0066]
2-苯基-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0067]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-苯基-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物56mg,产率72%,dr=3:1。
[0068][0069]
主要异构体:1h nmr(400mhz,cdcl3)δ8.05
–
7.95(m,4h),7.56
–
7.40(m,6h),7.29
–
7.22(m,5h),7.03(dd,j=10.2,3.4hz,1h),6.15(dd,j=10.2,1.1hz,1h),5.71(s,1h),5.59(dd,j=3.3,1.0hz,1h).
[0070]
次要异构体:1h nmr(400mhz,cdcl3)δ8.05
–
7.95(m,4h),7.56
–
7.40(m,6h),7.29
–
7.22(m,5h),7.19(dd,j=10.2,1.4hz,1h),6.21(dd,j=10.2,1.9hz,1h),5.80(dd,j=3.3,1.6hz,1h),4.94(d,j=1.2hz,1h).
[0071]
13
c nmr(151mhz,cdcl3)δ195.28,194.28,152.70,150.06,141.19,140.92,140.37,140.00,135.69,135.57,133.03,132.95,132.85,132.78,129.34,129.27,129.20,129.11,128.72,128.19,128.14,128.09,127.99,127.93,127.91,127.84,127.66,127.58,125.77,124.81,81.50,81.49,77.72,77.52.高分辨质谱(esi,m/z):[m h]
calcd for c
23h20
no3s,390.1158;测量值:390.1158.
[0072]
实施例9:
[0073]
2-(对甲苯基)-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0074]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-(对甲苯基)-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物63mg,产率78%,dr=3:1。
[0075][0076]
主要异构体:1h nmr(400mhz,cdcl3)δ8.06
–
7.99(m,4h),7.56
–
7.42(m,6h),7.16
–
7.07(m,4h),7.03(dd,j=10.2,3.4hz,1h),6.15(dd,j=10.1,1.1hz,1h),5.69(s,1h),5.58(dd,j=3.3,1.1hz,1h),2.33(d,j=3.6hz,3h).
[0077]
次要异构体:1h nmr(400mhz,cdcl3)δ8.05
–
7.98(m,4h),7.56
–
7.42(m,6h),7.21
–
7.18(m,1h),7.16
–
7.07(m,4h),6.21(dd,j=10.2,1.9hz,1h),5.79(dd,j=3.3,1.6hz,1h),4.93(d,j=1.1hz,1h),2.33(d,j=3.6hz,3h).
[0078]
13
c nmr(151mhz,cdcl3)δ195.54,194.56,152.67,150.09,141.10,140.94,140.36,140.06,137.82,137.62,133.00,132.95,132.84,132.79,132.61,129.33,129.28,129.18,129.13,128.95,128.79,128.66,128.14,128.01,127.93,127.79,127.61,127.58,125.79,81.51,81.48,77.65,77.53,21.24,21.21.高分辨质谱(esi,m/z):[m h]
理论值:c
24h22
no3s,404.1314;测量值:404.1311.
[0079]
实施例10:
[0080]
2-(4-氯苯基)-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0081]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-(4-氯苯基)-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物63mg,产率75%,dr=3:1。
[0082][0083]
主要异构体:1h nmr(400mhz,cdcl3)δ8.04
–
7.95(m,4h),7.55
–
7.40(m,6h),7.29
–
7.17(m,4h),7.09
–
7.00(m,1h),6.13(dd,j=10.2,1.0hz,1h),5.69(s,1h),5.60(dd,j=3.5,1.0hz,1h).
[0084]
次要异构体:1h nmr(400mhz,cdcl3)δ8.04
–
7.95(m,4h),7.55
–
7.40(m,6h),7.29
–
7.17(m,4h),7.09
–
7.00(m,1h),6.20(dd,j=10.2,1.9hz,1h),5.82(dd,j=3.3,1.7hz,1h),4.92(d,j=1.0hz,1h).
[0085]
13
c nmr(151mhz,cdcl3)δ194.80,193.72,152.80,149.93,141.39,140.86,140.42,139.79,134.15,134.09,133.90,133.74,133.09,133.00,132.91,132.80,129.39,129.29,129.21,129.14,128.88,128.64,128.59,128.29,128.13,128.03,127.93,127.39,125.59,124.81,81.56,80.56,77.92,76.41.高分辨质谱(esi,m/z):[m h]
理论值:c
23h19
clno3s,424.0768;测量值:424.0755.
[0086]
实施例11:
[0087]
2-((叔丁基二甲基硅基)氧基)甲基)-6-(oxo-(二苯基-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0088]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二苯基亚砜亚胺(0.4mmol)和2-((叔丁基二甲基硅基)氧基)甲基)-6-羟基-2h-吡喃-3(6h)-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到无色液体产物46mg,产率50%,dr=6:1。
[0089][0090]
主要异构体:1h nmr(400mhz,cdcl3)δ8.07
–
8.00(m,4h),7.56
–
7.47(m,6h),6.96(dd,j=10.2,3.4hz,1h),6.05(dd,j=10.2,0.9hz,1h),5.65(dd,j=3.4,1.0hz,1h),4.72(dd,j=4.6,2.6hz,1h),4.03
–
3.91(m,2h),0.87
–
0.82(m,9h),0.05
–
0.03(m,6h).
[0091]
次要异构体:1h nmr(400mhz,cdcl3)δ8.07
–
8.00(m,4h),7.56
–
7.47(m,6h),7.03(dd,j=10.2,1.7hz,1h),6.11(dd,j=16.6,6.1hz,1h),5.79(d,j=2.9hz,1h),4.38(t,j=2.2hz,1h),4.03
–
3.91(m,2h),0.87
–
0.82(m,9h),0.05
–
0.03(m,6h).
[0092]
13
c nmr(101mhz,cdcl3)δ195.63,150.17,140.28,140.15,132.96,132.92,132.59,129.29,129.26,129.15,128.94,128.22,127.92,125.98,77.89,63.38,25.98,25.86,25.64,18.29,-5.42.高分辨质谱(esi,m/z):[m h]
理论值:c
24h32
no4ssi,458.1816;测量值:458.1815.
[0093]
实施例12:
[0094]
6-((二甲基(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0095]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二甲基亚砜亚胺(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到无色液体产物29mg,产率77%。
[0096][0097]1h nmr(400mhz,cdcl3)δ6.94(dd,j=10.3,2.9hz,1h),6.08(dd,j=10.3,1.5hz,1h),5.64(dd,j=2.8,1.4hz,1h),4.45(d,j=16.4hz,1h),4.08(d,j=16.4hz,1h),3.15(d,j=0.7hz,3h),3.08(d,j=0.6hz,3h).
[0098]
13
c nmr(101mhz,cdcl3)δ194.90,150.80,126.37,78.04,67.58,44.61,43.69.高分辨质谱(esi,m/z):[m h]
理论值:c7h
12
no3s,190.0532;测量值:190.0535.
[0099]
实施例13:
[0100]
6-((二丁基(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0101]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二丁基亚砜亚胺(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物32mg,产率58%。
[0102][0103]1h nmr(400mhz,cdcl3)δ6.94(dd,j=10.3,2.9hz,1h),6.08(dd,j=10.3,1.4hz,1h),5.61(dd,j=2.6,1.3hz,1h),4.47(d,j=16.4hz,1h),4.07(d,j=16.4hz,1h),3.25
–
2.96(m,4h),1.88
–
1.73(m,4h),1.52
–
1.41(m,4h),0.97(dd,j=7.4,6.7hz,6h).
13
c nmr(101mhz,cdcl3)δ195.20,151.41,126.16,77.74,67.72,54.86,52.11,25.55,23.62,21.85,21.67,13.60,13.57.高分辨质谱(esi,m/z):[m na]
理论值:c
13h23
no3sna,296.1291;测量值:296.1295.
[0104]
实施例14:
[0105]
6-((二苄基(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0106]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加二苄基亚砜亚胺(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到无色液体产物35mg,产率52%。
[0107][0108]1h nmr(400mhz,cdcl3)δ7.47
–
7.32(m,10h),6.74(dd,j=10.3,2.8hz,1h),6.03(dd,j=10.3,0.9hz,1h),5.44(s,1h),4.47(d,j=13.9hz,1h),4.39(dd,j=18.7,15.2hz,2h),4.15(s,2h),4.02(d,j=16.5hz,1h).
13
c nmr(151mhz,cdcl3)δ195.27,151.36,131.49,131.03,129.18,129.05,128.96,128.65,128.60,126.79,126.04,78.05,67.94,62.11,58.53.高分辨质谱(esi,m/z):[m na]
理论值:c
19h19
no3sna,364.0978;测量值:364.0980.
[0109]
实施例15:
[0110]
6-((甲基(oxo)(苯基)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0111]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加甲基(亚氨基)(苯基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到无色液体产物33mg,产率66%,dr=1:1。
[0112][0113]
异构体一:1h nmr(400mhz,cdcl3)δ8.00
–
7.96(m,2h),7.69
–
7.56(m,3h),6.93(ddd,j=11.6,10.4,3.1hz,1h),6.10
–
6.05(m,1h),5.45
–
5.40(m,1h),4.64(d,j=16.6hz,1h),3.91(d,j=16.5hz,1h),3.20(s,3h).
[0114]
异构体二:1h nmr(400mhz,cdcl3)δ8.00
–
7.96(m,2h),7.69
–
7.56(m,3h),6.93(ddd,j=11.6,10.4,3.1hz,1h),6.10
–
6.05(m,1h),5.45
–
5.40(m,1h),4.46(d,j=16.5hz,1h),4.13
–
4.07(m,1h),3.15(s,3h).
[0115]
13
c nmr(101mhz,cdcl3)δ195.22,195.15,150.87,150.12,140.11,138.82,133.61,133.46,129.65,129.46,128.24,127.97,126.24,125.80,78.53,77.83,67.59,67.50,45.95,45.62.高分辨质谱(esi,m/z):[m h]
理论值:c
12h14
no3s,252.0688;测量值:252.0694.
[0116]
实施例16:
[0117]
6-((乙基(oxo)(苯基)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0118]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加乙基(亚氨基)(苯基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到无色液体产物25mg,产率48%,dr=1:1。
[0119][0120]
异构体一:1h nmr(400mhz,cdcl3)δ7.99
–
7.91(m,2h),7.70
–
7.56(m,3h),6.95(ddd,j=11.9,10.3,3.1hz,1h),6.07(ddd,j=10.3,2.2,1.3hz,1h),5.44(ddd,j=12.0,3.1,1.1hz,1h),4.66(d,j=16.6hz,1h),3.93(d,j=16.5hz,1h),3.42
–
3.19(m,2h),1.32
–
1.21(m,3h).
[0121]
异构体二:1h nmr(400mhz,cdcl3)δ7.99
–
7.91(m,2h),7.70
–
7.56(m,3h),6.95(ddd,j=11.9,10.3,3.1hz,1h),6.07(ddd,j=10.3,2.2,1.3hz,1h),5.44(ddd,j=12.0,3.1,1.1hz,1h),4.48(d,j=16.5hz,1h),4.09(d,j=16.6hz,1h),3.42
–
3.19(m,2h),1.32
–
1.21(m,3h).
[0122]
13
c nmr(151mhz,cdcl3)δ195.41,195.29,151.21,150.34,138.13,136.67,133.55,133.42,129.55,129.35,129.03,128.82,126.09,125.67,78.49,77.84,67.62,67.52,51.79,51.44,7.58,7.01.高分辨质谱(esi,m/z):[m na]
理论值:c
13h15
no3sna,288.0665;测量值:288.0670.
[0123]
实施例17:
[0124]
6-((4-(叔丁基)苯基)(oxo)(苯基)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0125]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加(4-(叔丁基)苯基)(亚氨基)(苯基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物38mg,产率51%,dr=1:1。
[0126][0127]
异构体一:1hnmr(400mhz,cdcl3)δ8.05
–
7.89(m,4h),7.56
–
7.49(m,5h),7.02(t,j=3.2hz,1h),6.10(s,1h),5.46(td,j=3.3,1.1hz,1h),4.74(d,j=9.0hz,1h),4.05(d,j=7.0hz,1h),1.30(t,j=2.3hz,9h).
[0128]
异构体二:1h nmr(400mhz,cdcl3)δ8.05
–
7.89(m,4h),7.56
–
7.49(m,5h),7.00(t,j=3.2hz,1h),6.07(s,1h),5.46(td,j=3.3,1.1hz,1h),4.70(d,j=9.1hz,1h),4.09(d,j=7.0hz,1h),1.30(t,j=2.3hz,9h).
[0129]
13
c nmr(151mhz,cdcl3)δ195.50,157.00,156.96,150.64,140.59,140.24,136.91,132.91,132.87,129.31,129.26,128.56,128.53,128.14,128.05,126.45,126.42,125.72,125.67,78.39,78.36,67.49,67.47,35.16,31.03.高分辨质谱(esi,m/z):[m h]
理论值:c
21h24
no4s,370.1471;测量值:370.1469.
[0130]
实施例18:
[0131]
6-((甲基(oxo)(对甲苯基)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0132]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加亚氨基(甲基)(对甲苯基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物29mg,产率54%,dr=1:1。
[0133][0134]
异构体一:1h nmr(400mhz,cdcl3)δ7.91
–
7.83(m,2h),7.40(t,j=8.1hz,2h),6.99
–
6.90(m,1h),6.09(dd,j=4.7,1.2hz,1h),5.41(dd,j=3.1,1.1hz,1h),4.65(d,j=16.6hz,1h),3.93(d,j=16.5hz,1h),3.20(s,3h),2.46(t,j=3.9hz,3h).
[0135]
异构体二:1h nmr(400mhz,cdcl3)δ7.91
–
7.83(m,2h),7.40(t,j=8.1hz,2h),6.99
–
6.90(m,1h),6.06(dd,j=4.7,1.2hz,1h),5.41(dd,j=3.1,1.1hz,1h),4.49(d,j=16.5hz,1h),4.09(d,j=16.6hz,1h),3.15(s,3h),2.46(t,j=3.9hz,3h).
[0136]
13
c nmr(151mhz,cdcl3)δ195.31,195.23,151.01,150.22,144.67,144.48,136.78,135.54,130.29,130.10,128.31,128.04,126.16,125.69,78.56,77.81,77.30,77.09,76.88,67.55,67.45,45.94,45.68,21.56.高分辨质谱(esi,m/z):[m na]
理论值:c
13h15
no3sna,288.0665;测量值:288.0670.
[0137]
实施例19:
[0138]
6-((4-甲氧基苯基)(甲基)(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0139]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加亚氨基(4-甲氧苯基)(甲基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物25mg,产率45%,dr=1:1。
[0140][0141]
异构体一:1h nmr(600mhz,cdcl3)δ7.88(dd,j=11.3,9.0hz,2h),7.03(dd,j=10.1,9.1hz,2h),6.94
–
6.89(m,1h),6.08
–
6.03(m,1h),5.38(dd,j=9.4,1.7hz,1h),4.62(d,j=16.6hz,1h),3.92(d,j=16.5hz,1h),3.87(d,j=2.4hz,3h),3.17(s,3h).
[0142]
异构体二:1h nmr(600mhz,cdcl3)δ7.88(dd,j=11.3,9.0hz,2h),7.03(dd,j=10.1,9.1hz,2h),6.94
–
6.89(m,1h),6.08
–
6.03(m,1h),5.38(dd,j=9.4,1.7hz,1h),4.47(d,j=16.5hz,1h),4.09
–
4.05(m,1h),3.87(d,j=2.4hz,3h),3.12(s,3h).
[0143]
13
c nmr(151mhz,cdcl3)δ195.35,195.25,163.75,163.67,151.15,150.27,130.54,130.20,126.18,125.68,114.88,114.67,78.53,77.84,67.63,67.46,55.77,55.72,46.16,45.94.高分辨质谱(esi,m/z):[m h]
理论值:c
13h16
no4s,282.0794;测量值:
282.0802.
[0144]
实施例20:
[0145]
6-((4-氯苯基)(甲基)(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0146]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加亚氨基(4-氯苯基)(甲基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物30mg,产率53%,dr=1:1。
[0147][0148]
异构体一:1hnmr(600mhz,cdcl3)δ7.90(dd,j=8.5,3.0hz,2h),7.55(dd,j=13.9,8.6hz,2h),6.90(ddd,j=15.5,10.3,3.0hz,1h),6.08
–
6.05(m,1h),5.40(dd,j=10.8,1.5hz,1h),4.59(d,j=16.6hz,1h),3.91(d,j=16.5hz,1h),3.19(s,3h).
[0149]
异构体二:1h nmr(600mhz,cdcl3)δ7.90(dd,j=8.5,3.0hz,2h),7.55(dd,j=13.9,8.6hz,2h),6.90(ddd,j=15.5,10.3,3.0hz,1h),6.08
–
6.05(m,1h),5.40(dd,j=10.8,1.5hz,1h),4.41(d,j=16.5hz,1h),4.08(d,j=16.6hz,1h),3.13(s,3h).
[0150]
13
c nmr(151mhz,cdcl3)δ195.02,194.91,150.67,149.93,140.41,140.23,138.70,137.42,129.94,129.77,129.74,129.45,126.30,125.94,78.47,77.72,67.65,67.54,46.05,45.64.高分辨质谱(esi,m/z):[m na]
理论值:c
12h12
clno3sna,308.0118;测量值:308.0121.
[0151]
实施例21:
[0152]
6-((4-溴苯基)(甲基)(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0153]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加亚氨基(4-溴苯基)(甲基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物39mg,产率59%,dr=1:1。
[0154][0155]
异构体一:1h nmr(400mhz,cdcl3)δ7.84(dd,j=8.6,2.4hz,2h),7.73(t,j=8.7hz,2h),6.91(td,j=10.7,3.0hz,1h),6.09(d,j=2.4hz,1h),5.43(d,j=2.7hz,1h),4.60(d,j=16.6hz,1h),3.92(d,j=16.5hz,1h),3.20(s,3h).
[0156]
异构体二:1h nmr(400mhz,cdcl3)δ7.84(dd,j=8.6,2.4hz,2h),7.73(t,j=8.7hz,2h),6.91(td,j=10.7,3.0hz,1h),6.07(d,j=2.3hz,1h),5.41(d,j=2.5hz,1h),4.42(d,j=16.5hz,1h),4.12
–
4.07(m,1h),3.14(s,3h).
[0157]
13
c nmr(151mhz,cdcl3)δ195.03,194.92,150.68,149.93,139.29,138.01,132.95,132.75,129.86,129.52,128.96,128.80,126.33,125.97,78.49,77.74,67.69,67.57,46.05,45.65.高分辨质谱(esi,m/z):[m na]
理论值:c
12h12
brno3sna,351.9613;测量值:351.9620.
[0158]
实施例22:
[0159]
6-((3-甲氧基苯基)(甲基)(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0160]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加亚氨基(3-甲氧基苯基)(甲基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物33mg,产率58%,dr=1:1。
[0161][0162]
异构体一:1hnmr(600mhz,cdcl3)δ7.55
–
7.45(m,3h),7.20
–
7.12(m,1h),6.93(dd,j=10.3,3.0hz,1h),6.08
–
6.06(m,1h),5.41(d,j=1.7hz,1h),4.62(d,j=16.6hz,1h),3.92(d,j=16.5hz,1h),3.87(s,3h),3.19(s,3h).
[0163]
异构体二:1h nmr(600mhz,cdcl3)δ7.55
–
7.45(m,3h),7.20
–
7.12(m,1h),6.90(dd,j=10.3,3.1hz,1h),6.05(dd,j=4.6,0.9hz,1h),5.39(d,j=1.8hz,1h),4.46(d,j=16.5hz,1h),4.08(d,j=16.6hz,1h),3.86(s,3h),3.14(s,3h).
[0164]
13
c nmr(151mhz,cdcl3)δ195.24,195.24,195.14,160.41,160.21,150.88,150.09,141.24,139.90,130.71,130.49,126.22,125.76,120.26,119.85,119.82,112.93,112.87,78.61,77.78,67.59,67.49,55.78,55.72,45.86,45.60.高分辨质谱(esi,m/z):[m na]
理论值:c
13h15
no4sna,304.0614;测量值:304.0619.
[0165]
实施例23:
[0166]
6-((3-溴苯基)(甲基)(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0167]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加亚氨基(3-溴苯基)(甲基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物42mg,产率64%,dr=1:1。
[0168][0169]
异构体一:1hnmr(400mhz,cdcl3)δ8.11(dt,j=3.6,1.8hz,1h),7.90(ddd,j=7.9,3.3,1.8hz,1h),7.77(dddd,j=9.0,8.0,1.8,0.9hz,1h),7.46(dt,j=9.7,7.9hz,1h),6.95
–
6.88(m,1h),6.09(dd,j=4.5,1.3hz,1h),5.42(dt,j=2.9,1.4hz,1h),4.59(d,j=
16.6hz,1h),4.09(d,j=16.6hz,1h),3.20(s,3h).
[0170]
异构体二:1h nmr(400mhz,cdcl3)δ8.11(dt,j=3.6,1.8hz,1h),7.90(ddd,j=7.9,3.3,1.8hz,1h),7.77(dddd,j=9.0,8.0,1.8,0.9hz,1h),7.46(dt,j=9.7,7.9hz,1h),6.95
–
6.88(m,1h),6.06(dd,j=4.5,1.3hz,1h),5.42(dt,j=2.9,1.4hz,1h),4.42(d,j=16.5hz,1h),3.92(d,j=16.5hz,1h),3.15(s,3h).
[0171]
13
c nmr(151mhz,cdcl3)δ195.00,194.92,150.51,149.86,142.38,140.96,136.68,136.49,131.21,131.10,130.92,130.90,126.68,126.44,126.33,125.97,123.66,123.40,78.48,77.65,67.56,46.03,45.56.高分辨质谱(esi,m/z):[m na]
理论值:c
12h12
brno3sna,351.9613;测量值:351.9620.
[0172]
实施例24:
[0173]
6-((2-甲氧基苯基)(甲基)(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0174]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加亚氨基(2-甲氧基苯基)(甲基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物25mg,产率44%,dr=1:1。
[0175][0176]
异构体一:1hnmr(400mhz,cdcl3)δ8.11
–
7.99(m,1h),7.68
–
7.57(m,1h),7.21
–
7.04(m,2h),6.94(dd,j=10.2,3.5hz,1h),6.03(s,1h),5.41(d,j=3.3hz,1h),4.67(d,j=16.6hz,1h),3.99(s,3h),3.75(d,j=16.4hz,1h),3.35(s,3h).
[0177]
异构体二:1h nmr(400mhz,cdcl3)δ8.11
–
7.99(m,1h),7.68
–
7.57(m,1h),7.21
–
7.04(m,2h),6.83(dd,j=10.2,3.3hz,1h),6.01(s,1h),5.19(d,j=3.0hz,1h),4.45(d,j=16.4hz,1h),4.04(d,j=16.6hz,1h),3.98(s,3h),3.34(s,3h).
[0178]
13
c nmr(151mhz,cdcl3)δ195.70,195.58,156.90,156.63,150.62,150.08,135.70,135.30,131.97,130.75,126.03,125.55,121.25,120.96,112.20,112.07,79.04,76.90,67.20,66.70,56.10,56.06,43.69,43.43.高分辨质谱(esi,m/z):[m h]
理论值:c
13h16
no4s,282.0794;测量值:282.0798.
[0179]
实施例25:
[0180]
6-((2-氯苯基)(甲基)(oxo)-λ-亚砜基)氨基)-2h-吡喃-3(6h)-酮的制备
[0181]
在空气下,将(pho)2p(o)oh(0.04mmol)溶于chcl3(2ml),添加亚氨基(2-氯苯基)(甲基)-λ-磺胺酮(0.4mmol)和6-羟基-2h-吡喃-3-酮(0.2mmol)于混合物中。将混合物在室温下搅拌18h,旋干后柱层析分离得到黄色液体产物34mg,产率60%,dr=1:1。
[0182]
[0183]
异构体一:1h nmr(400mhz,cdcl3)δ8.22(dt,j=16.8,5.0hz,1h),7.65
–
7.43(m,3h),6.90(d,j=3.4hz,1h),6.04(s,1h),5.42(d,j=3.4hz,1h),4.63(d,j=16.7hz,1h),3.83(d,j=16.6hz,1h),3.38(s,3h).
[0184]
异构体二:1h nmr(400mhz,cdcl3)δ8.22(dt,j=16.8,5.0hz,1h),7.65
–
7.43(m,3h),6.88(d,j=3.4hz,1h),6.01(s,1h),5.12(d,j=3.2hz,1h),4.51(d,j=16.6hz,1h),4.02(d,j=16.7hz,1h),3.38(s,3h).
[0185]
13
c nmr(151mhz,cdcl3)δ195.27,195.19,150.01,149.51,138.14,134.89,134.85,134.43,133.24,132.31,132.15,132.08,131.97,131.73,128.11,127.67,126.18,125.73,79.16,77.00,67.37,67.36,43.44,43.23.高分辨质谱(esi,m/z):[m na]
理论值:c
12h12
clno3sna,308.0118;测量值:308.0119.
[0186]
上述实施例为本发明较佳的实施方式,但本发明的实施方式并不受上述实施例的限制,其他的任何未背离本发明的精神实质与原理下所作的改变、修饰、替代、组合、简化,均应为等效的置换方式,都包含在本发明的保护范围之内。
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。