1.本发明属于有机合成技术领域,涉及3-氰烷基-4-吡咯啉-2-酮及其衍生物,具体涉及光催化合成3-氰烷基-4-吡咯啉-2-酮及其衍生物的方法。
背景技术:
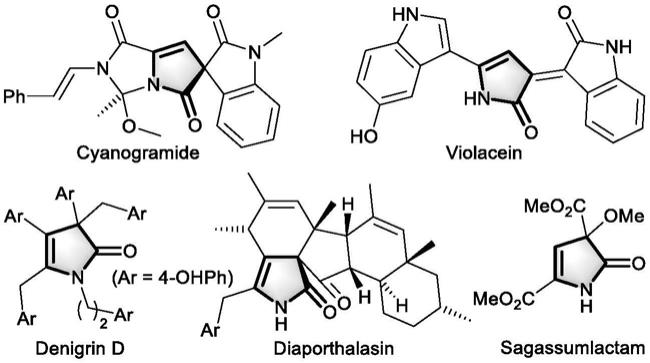
2.4-吡咯啉-2-酮(又称1,3-二氢吡咯-2-酮和β,γ-不饱和-γ-丁内酰胺)广泛存在于如cyanogramide、violacein、denigrin、diaporthalasin、sagassumlactam等天然生物碱或临床药物分子中。该杂环结构的重要性持续激励着人们开发它的构建方法,但现有的方法通常都需要苛刻的反应条件(z.zhao,x.kong,w.wang,j.hao and y.wang,direct use of unprotected aliphatic amines to generate n-heterocycles viaβ-c-h malonylation with iodonium ylide,org.lett.,2020,22,230-233)、贵重金属催化剂(a.n.koronatov,n.v.rostovskii,a.f.khlebnikov and m.s.novikov,synthesis of 3-alkoxy-4-pyrrolin-2-ones via rhodium(ii)-catalyzed denitrogenative transannulation of 1h-1,2,3-triazoles with diazo esters,org.lett.,2020,22,7958-7963)或制备复杂的底物。因此,从易得原料出发开发温和、经济、绿色的官能团化4-吡咯啉-2-酮及其衍生物的合成方法仍然十分必要。
[0003][0004]
部分含4-吡咯啉-2-酮的生物碱
技术实现要素:
[0005]
为了解决现有技术中的问题,本发明提供一种光催化合成3-氰烷基-4-吡咯啉-2-酮及其衍生物的方法。
[0006]
为实现上述目的,本发明的技术方案为:
[0007]
3-氰烷基-4-吡咯啉-2-酮及其衍生物的合成方法,其特征在于:以3-氮杂-1,5-二烯衍生物(式1)和肟酯衍生物(式2)为起始原料,光催化合成3-氰烷基-4-吡咯啉-2-酮及其衍生物(式3),反应路线如下:
[0008][0009]
其中:
[0010]
r1为h、c
1-6
烷基、苯基或-(ch2)ncn;
[0011]
r2为
[0012]
r3为c
1-6
烷基、苯基或-(ch2)
m-r9;
[0013]
r4为h或c
1-6
烷基;
[0014]
r5、r6各自独立为h或-coo c
1-6
烷基;
[0015]
r7为h、一个或多个取代的c
1-6
烷基、卤素或卤代c
1-6
烷基;
[0016]
r9为-o-c
1-6
烷基、-n-c
1-6
烷基、n位取代吗啉基、-ch=ch2或-c≡ch;
[0017]
r8为h、一个或多个卤素取代的c
1-6
烷基、卤素或-o-c
1-6
烷基;
[0018]
n=1-4;m=1-4。
[0019]
本发明肟酯衍生物(式2)的ar(芳基)优选4-cf3ph、2,4-cl2ph或4-meo ph。
[0020]
本文所用的术语“卤素”是指氟、氯、溴或碘。本文所用的术语“c
1-6
烷基或环烷基”是指具有1-6个碳原子的饱和的直链或支链烃基或环烷基,例如甲基、乙基、丙基、异丙基、正丁基、异丁基、仲丁基、叔丁基、正戊基、异戊基、新戊基、正己基、异己基等,优选甲基、乙基、丙基、异丙基、叔丁基、异丁基、环丙基、环戊基或环己基。本发明所用的术语
“‑
o-c
1-6
烷基”,是指具有1-6个碳原子的饱和的直链或支链烷氧基,例如甲氧基、乙氧基、叔丁氧基等。本文所用的术语“卤素取代的c
1-6
烷基”指的是一个或多个卤素取代的具有1-6个碳原子的饱和的直链或支链烃基,比如二氟甲基、三氟甲基等。
[0021]
本文化合物中,r7可以独立的为邻位、间位或对位,可以邻位、间位或/和对位同时取代,也可以单独取代。
[0022]
本文化合物中,r8可以独立的为邻位、间位或对位,可以邻位、间位或/和对位同时取代,也可以单独取代。
[0023]
优选的,上述方法中,r1为h、me、et、pr或ph;r2为r3为bn、pr、cy或ph;r4为h或me;r5、r6各自独立为h、-co2me或-co2ipr;r7为4-me、4-et、4-meo、4-bno、4-br、4-cl、4-f、4-cf3、4-ph、3-cl、3-cf3、3,5-(cf3)2或2,4-cl2。
[0024]
本发明光催化的催化剂剂选自ir(ppy)3、[ir(dtbbpy)(ppy)2]pf6、ru(bpy)3cl2、4czipn,用量优选0.5-1mol%。本发明溶剂选自ch3cn、dce、甲苯、二甲亚砜或丙酮。本发明光为蓝光或太阳自然光。本发明式1化合物的用量为1-1.5当量。
[0025]
本发明所制备的3-氰烷基-4-吡咯啉-2-酮及其衍生物(化合物3)选自:
[0026]
3a,5-(1-苄基-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0027]
3b1,5-(1-苄基-4-乙基-3-甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0028]
3b2,5-(1-苄基-3-甲基-2-氧代-5-苯基-4-丙基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0029]
3b3,5-(1-苄基-3-甲基-2-氧代-4,5-二苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0030]
3c,5-(1-苄基-4-(3-氰丙基)-3-甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0031]
3d1,5-(1-苄基-3,4-二甲基-2-氧代-5-(对甲苯基)-2,3-二氢-1h-吡咯-3-基)戊腈,
[0032]
3d2,5-(1-苄基-5-(4-乙基苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0033]
3d3,5-(1-苄基-5-(4-甲氧基苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0034]
3d4,5-(1-苄基-5-(4-(苄氧基)苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0035]
3d5,5-(1-苄基-5-(4-溴苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0036]
3d6,5-(1-苄基-5-(4-氯苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0037]
3d7,5-(1-苄基-5-(4-氟苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0038]
3d8,5-(1-苄基-3,4-二甲基-2-氧代-5-(4-(三氟甲基)苯基)-2,3-二氢-1h-吡咯-3-基)戊腈,
[0039]
3d9,5-(5-([1,1'-连苯基]-4-基)-1-苄基-4-乙基-3-甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0040]
3d10,5-(1-苄基-5-(3-氯苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0041]
3d11,5-(1-苄基-3,4-二甲基-2-氧代-5-(3-(三氟甲基)苯基)-2,3-二氢-1h-吡咯-3-基)戊腈,
[0042]
3d12,5-(1-苄基-5-(3,5-双(三氟甲基)苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0043]
3d13,5-(1-苄基-5-(2,4-二氯苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,
[0044]
3e,5-(1-苄基-4-(3-氰丙基)-3-甲基-2-氧代-5-(吡啶-2-基)-2,3-二氢-1h-吡咯-3-基)戊腈,
[0045]
3e',n-苄基-n-(5-氰基-1-(吡啶-2-基)戊-1-烯-1-基)甲基丙烯酰胺,
[0046]
3f,5-(1-苄基-3-甲基-2-氧代-2,3,4,5,6,7-六氢-1h-吲哚-3-基)戊腈,
[0047]
3g1,5-(3,4-二甲基-2-氧代-5-苯基-1-丙基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0048]
3g2,5-(1-环己基-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0049]
3g3,5-(3,4-二甲基-2-氧代-1,5-二苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0050]
3h,5-(1-(2-甲氧基乙基)-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0051]
3i,5-(3,4-二甲基-1-(2-吗啉基乙基)-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0052]
3j1,5-(4-乙基-1-(3-甲氧基苄基)-3-甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0053]
3j2,5-(3,4-二甲基-1-(4-甲基苄基)-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0054]
3j3,5-(1-(4-氯苄基)-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0055]
3j4,5-(1-(4-氟苄基)-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0056]
3k,5-(1-烯丙基-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0057]
3l,5-(3,4-二甲基-2-氧代-5-苯基-1-(丙-2-炔-1-基)-2,3-二氢-1h-吡咯-3-基)戊腈,
[0058]
3m,5-(1-(4-氯苄基)-4-甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,
[0059]
3n,4-(1-(4-氯苄基)-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)-2-(氰甲基)丁酸甲酯,
[0060]
3o,2-(2-(1-(4-氯苄基)-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)乙基)-2-(氰甲基)丙二酸二异丙酯。
[0061]
本发明提供上述3-氰烷基-4-吡咯啉-2-酮及其衍生物在作为合成如cyanogramide、violacein、denigrin、diaporthalasin或sagassumlactam的原料或者作为这些药物及其衍生物的杂质对照品。
[0062]
有益效果:
[0063]
过去的十多年里,烯烃官能团化反应已被证明是快速构建复杂分子的强力手段,人们在该领域投入了大量研究精力。π键的键能很低,它与亲电体或自由基反应时通常会发生π键断裂。另一方面,烯酰胺是一类具有高共轭能的富电子烯烃,也是大量生物活性分子的合成中间体,近年来获得了广泛关注,人们通过其β-c(sp2)-h键官能团化反应合成了大量β-官能团化的烯酰胺。由于π键的活性,烯烃无论是贫电子的还是富电子的,活化的还是非活化的,遇上自由基时都会呈现高度的反应活性。因此,当一个分子中有2个烯基时,由于2个双键都能充当自由基受体反应将存在选择性问题(t.-y.zhao,l.-j.xiao and q.-l.zhou,nickel-catalyzed desymmetric reductive cyclization/coupling of 1,6-dienes:an enantioselective approach to chiral tertiary alcohol,angew.chem.,int.ed.,2022,61)。
[0064]
本发明提供一种光催化合成3-氰烷基-4-吡咯啉-2-酮及其衍生物的方法。出乎意料的是,本发明使用n-烯基丙烯酰胺与环丁酮肟酯在可见光的诱导作用下发生氰烷基化/环化串联反应,成功合成了3-氰烷基-4-吡咯啉-2-酮及其衍生物,而且反应的底物适用范围广,区域专一性强。本发明方法具有区域专一光催化反应的实用性,还可以使用自然太阳光作为可持续的绿色光源,具有高度的生产实用性。
具体实施方式
[0065]
下面通过具体实施例对本发明进行具体描述,在此指出以下实施例只用于对本发明进行进一步说明,不能理解为对本发明保护范围的限制,本领域的技术熟练人员可以根据上述发明内容对本发明作出一些非本质的改进和调整。本发明所用原料及试剂均为市售产品。除特殊说明外,本发明所述份数均为重量份,所述百分比均为质量百分比。本发明实施例的底物n-烯基丙烯酰胺为3-氮杂-1,5-二烯衍生物。
[0066]
实施例
[0067]
产物合成步骤:
[0068]
3-氰烷基-4-吡咯啉-2-酮及其衍生物合成的一般步骤:
[0069]
氩气氛围下,向装有磁力搅拌子的25ml schlenk反应管中,依次加入肟酯2(0.3mmol)、n-烯基丙烯酰胺1(1.5当量,0.45mmol)和fac-ir(ppy)3(0.5mol%,0.0015mmol,1mg),然后加入除氧乙腈(2.0ml)。反应在无氧环境中进行。混合物在室温和蓝光led照射下搅拌12小时,然后加水(20.0ml)终止反应,并用ch2cl2(20.0ml
×
4)萃取四次。蒸干溶剂得到的残余物通过硅胶柱层析分离提纯(石油醚-乙酸乙酯体积比为21:1),得到产物3-氰烷基-4-吡咯啉-2-酮3。
[0070]
底物n-烯基丙烯酰胺1合成
[0071]
底物1按文献方法合成(m.kobayashi,t.suda,k.noguchi and k.tanaka,angew.chem.,int.ed.,2011,50,1664
–
1667)。
[0072]
反应路线为:
[0073][0074]
反应步骤为:甲苯(14ml)的伯胺(13.9mmol)、酮(13.9mmol)和分子筛(4.5g)的混合溶液在120℃反应6h,得到粗亚胺的混合溶液。冷却后,滤除分子筛,再用甲苯(3ml)洗涤。冰水浴冷却下向滤液中加入三乙胺(2当量,27.8mmol)和甲基丙烯酰氯(1.2当量,16.7mmol),然后自然恢复到室温并继续在室温搅拌12小时。用水(50ml)淬灭反应,并用ch2cl2(25ml
×
4)萃取。蒸干溶剂得到的残余物通过硅胶柱层析分离提纯(石油醚-乙酸乙酯),得到n-烯基丙烯酰胺1。
[0075]
底物环酮肟酯2合成
[0076]
底物2按文献方法合成(x.-y.yu,j.-r.chen,p.-z.wang,m.-n.yang,d.liang and w.-j.xiao,angew.chem.,int.ed.,2018,57,738
–
743)
[0077][0078]
向装有磁力搅拌子的烧瓶中加入酮(5.0mmol,1.0当量)和盐酸羟胺(5.5mmol,1.1当量),再加入饱和碳酸钠水溶液维持溶液的ph值在7-8(约10ml)并在40℃反应。反应结束
后,用ch2cl2萃取,蒸干有机溶剂得到粗品环酮肟,不经进一步纯化直接用于下一步反应。冰水浴冷却下,向环酮肟(1.0当量)、三乙胺(2.0当量)的ch2cl2(0.5m)溶液中加入对三氟甲基苯甲酰氯(1.5当量)。反应6小时后,加水终止,用ch2cl2萃取。蒸干溶剂得到的残余物通过硅胶柱层析分离提纯(石油醚-乙酸乙酯),得到环酮肟酯2。
[0079]
结果与讨论
[0080]
表1反应条件考察
[0081][0082][0083]
a 1
h nmr产率,均三甲氧基苯作为内标。b反应条件:1a(0.3mmol),2a(0.3mmol),fac-ir(ppy)3(0.003mmol),mecn(3.0ml),6w蓝光led(455nm),ar,室温,12h。
[0084]
n-烯基甲基丙烯酰胺1a和对三氟甲基苯甲酸环丁酮肟酯2a选为模型底物进行反应条件优化(表1)。以ir(ppy)3(1mol%)为光催化剂(pc),等摩尔比的1a和2a的乙腈(3ml)溶液在蓝光发光二极管(led)的照射下,发生反应生成目标产物3-氰烷基-4-吡咯啉-2-酮3a,1h nmr产率为50%(序号1)。发明人接着尝试了其他pc,包括[ir(dtbbpy)(ppy)2]pf6(序号2),ru(bpy)3cl2(序号3)和4czipn(序号4)等,但3a的产率都有所下降。将1,5-二烯1a的用量增加到1.5当量时,3a的nmr产率提高到68%(序号5)。碱(如k2co3,序号6)或酸(如hoac,序号7)等添加剂的加入对反应没有益处。反应受到溶剂效应的影响较小,无论是在非极性溶剂(如dce(序号8)和甲苯)还是在极性溶剂(如丙酮(序号9)和二甲亚砜(dmso))中进行,3a的产率都差不多。增加反应液浓度,将溶剂用量降低到2ml(0.15m)时,反应的nmr产率提升到74%(序号10)。发明人研究了肟酯苯甲酰基苯环上的取代基效应。供电子基不利于烷基
自由基生成,例如带有富电子苯甲酰基的肟酯1a参加反应时3a的产率较低(序号11)。用2,4-二氯苯甲酰氯合成的环丁酮肟酯也是可用的氰烷基自由基前体(序号12)。没有光照时,没有反应发生(序号13)。
[0085]
参照上述操作步骤,发明人研究了反应的适用范围(表2)。1,5-二烯烯胺部分2-位苯基上带有甲基、乙基、丙基或苯基时,都能顺利与肟酯2a发生反应生成对应4-吡咯啉-2-酮3a-3b3,分离产率60-78%。含有末端双键的苯乙烯基底物在2.5当量2a存在时,发生双氰烷基化/环化反应,生成双氰烷基化的4-吡咯啉-2-酮3c,反应48h后产率为36%。α-(2-吡啶基)的末端烯胺底物也和2当量2a反应生成双氰烷基化/环化产物3e,产率45%,同时还伴随着28%产率的单氰烷基化开链产物3e'生成。3e'由烯酰胺部分发生β-官能团化反应生成。这些结果表明,当反应涉及末端烯酰胺底物时,区域选择性可能下降甚至翻转。对位或间位取代的富电子(3d1-3d4)、电中性(3d9)和贫电子的1-芳基基团(3d10和3d11)都能被该反应兼容,对应的4-吡咯啉-2-酮产物都高产率生成。3,5-双三氟甲基和2,4-二氯取代的双取代1-芳基底物也能参与该反应,对应4-吡咯啉-2-酮产物3d11和3d12的产率分别为59%和70%。值得注意的是,由脂肪环己酮制备的共轭能较低的底物也能顺利与2a发生该环化反应,以65%的产率生成1,3,4,5,6,7-六氢吲哚-2-酮3f。对n-保护基(pg)的测试表明,无论是烷基(3g1和3g2,3h-3j4)还是芳基保护(3g3)的n-烯基丙烯酰胺都是高活性底物,对应的内酰胺产物产率42-74%。值得注意的是,n-pg为非活化烯基或炔基时,额外的双键和三键都能被反应兼容,对应的n-烯丙基和n-炔丙基内酰胺3k和3l顺利生成这些结果再次表明,该反应有惊人的区域选择性和化学选择性。烯酰胺部分用于稳定自由基中间体的甲基并非必不可少,3-位单取代的4-吡咯啉-2-酮产物3m以53%的产率生成。该环合反应也能拓展到其它环丁酮合成的肟酯,例如从3-氧代环丁烷-1-羧酸甲酯,3-氧代环丁烷-1,1-二羧酸二异丙酯和2-氧代-7-氮杂螺环[3.5]壬烷-7-羧酸叔丁酯合成的环丁酮肟酯都顺利与3-氮杂-1,5-二烯反应生成对应3-氰烷基-4-吡咯啉-2-酮3n-3o。
[0086]
表2底物范围a[0087][0088]a反应条件:1(0.45mmol),2(0.3mmol),fac-ir(ppy)3(0.0015mmol),mecn(2.0ml),6w蓝光led(455nm),ar,室温,12h。b1c(0.3mmol),2a(2.5当量),48h.c1e(0.3mmol),2a(2当
量)。dfac-ir(ppy)3(1mol%)。
[0089]
为了证明这个区域专一光催化反应的实用性,发明人开展了4mmol规模的克级反应。此外,该反应还可以使用自然太阳光作为可持续的绿色光源。两个反应中4-吡咯啉-2-酮产物的产率都仅有轻微下降,表明该反应具有高度的生产实用性。
[0090][0091]
克级规模合成和太阳光实验
[0092]
综上所述,本发明开发了一个可见光诱导的n-烯基丙烯酰胺和肟酯的区域专一性氰烷基化/环化反应,该反应条件温和、官能团耐受性好,为3-氰烷基-4-吡咯啉-2-酮合成提供了便捷方法。
[0093]
产物光谱表征
[0094][0095]
3a,5-(1-苄基-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),70%产率(76mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.38
–
7.31(m,3h),7.19
–
7.12(m,3h),7.10
–
7.05(m,2h),6.88
–
6.84(m,2h),4.59
–
4.50(m,2h),2.26(qt,j=16.9,7.1hz,2h),1.83(ddd,j=13.3,11.5,5.1hz,1h),1.64
–
1.51(m,6h),1.26(s,3h),1.25
–
1.11(m,2h).
13
c{1h}nmr(101mhz,cdcl3)δ182.4,137.7,137.0,130.3,129.9,128.6,128.4,128.2,127.7,127.1,119.5,119.1,51.2,44.1,35.1,25.4,23.8,22.3,17.0,8.8.hrms(esi-tof)calcd for c
24h27
n2o
([m h]
)359.2118.found 359.2116.
[0096][0097]
3b1,5-(1-苄基-4-乙基-3-甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),69%产率(77mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.39
–
7.31(m,3h),7.15(dddd,j=5.8,4.0,2.2,2.2hz,3h),7.10
–
7.07
(m,2h),6.87
–
6.83(m,2h),4.50(s,2h),2.34
–
2.17(m,2h),2.04(ddt,j=32.9,14.7,7.4hz,2h),1.85(ddd,j=13.3,11.8,5.0hz,1h),1.68
–
1.51(m,3h),1.31(s,3h),1.30
–
1.22(m,1h),1.19
–
1.08(m,1h),0.92(t,j=7.6hz,3h).
13
c{1h}nmr(101mhz,cdcl3)δ182.0,137.9,137.7,130.7,129.9,128.7,128.3,128.2,127.7,127.1,124.5,119.6,51.8,44.1,35.8,25.5,23.9,23.2,17.6,17.1,15.1.hrms(esi-tof)calcd for c
25h29
n2o
([m h]
)373.2274.found 373.2277.
[0098][0099]
3b2,5-(1-苄基-3-甲基-2-氧代-5-苯基-4-丙基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),78%产率(91mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.38
–
7.30(m,3h),7.15(dddd,j=5.7,4.0,2.1,2.1hz,3h),7.08
–
7.05(m,2h),6.87
–
6.82(m,2h),4.50(s,2h),2.34
–
2.17(m,2h),2.02(ddd,j=14.4,10.3,6.4hz,1h),1.94
–
1.80(m,2h),1.68
–
1.50(m,3h),1.40
–
1.21(m,6h),1.12(dddd,j=14.9,12.6,10.3,5.4hz,1h),0.76(t,j=7.3hz,3h).
13
c{1h}nmr(101mhz,cdcl3)δ182.1,138.3,137.7,130.7,130.0,128.6,128.3,128.2,127.7,127.1,123.1,119.6,51.7,44.1,35.8,26.9,25.5,23.9,23.4,23.1,17.1,14.5.hrms(esi-tof)calcd for c
26h31
n2o
([m h]
)387.2431.found 387.2443.
[0100][0101]
3b3,5-(1-苄基-3-甲基-2-氧代-4,5-二苯基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),60%产率(76mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.30
–
7.11(m,9h),7.07
–
7.03(m,2h),7.02
–
6.99(m,2h),6.94
–
6.89(m,2h),4.68(d,j=15.2hz,1h),4.62(d,j=15.2hz,1h),2.28
–
2.14(m,2h),1.87(ddd,j=13.3,11.8,4.9hz,1h),1.68
–
1.50(m,3h),1.49
–
1.36(m,4h),1.27
–
1.15(m,1h).
13
c{1h}nmr(101mhz,cdcl3)δ182.2,139.9,137.5,133.8,130.5,130.1,129.4,128.7,128.4,128.3,128.2,127.7,127.2,126.8,123.8,119.6,52.6,44.3,35.9,25.4,23.9,23.5,17.0.hrms(esi-tof)calcd for c
29h29
n2o
([m h]
)421.2274.found 421.2271.
[0102][0103]
3c,5-(1-苄基-4-(3-氰丙基)-3-甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),36%产率,无色油状物.1h nmr(400mhz,cdcl3)δ7.42
–
7.33(m,3h),7.17
–
7.14(m,3h),7.06(ddd,j=6.6,1.6,1.6hz,2h),6.85
–
6.82(m,2h),4.50(s,2h),2.36
–
2.06(m,6h),1.87(ddd,j=13.4,11.6,5.1hz,1h),1.67
–
1.51(m,5h),1.34
–
1.12(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ181.6,139.8,137.4,130.2,129.7,129.2,128.7,128.2,127.7,127.2,120.4,119.4,119.1,51.6,44.1,35.7,25.6,25.3,23.7,23.6,23.0,17.2,17.0.hrms(esi-tof)calcd for c
27h30
n3o
([m h]
)412.2383.found 412.2370.
[0104][0105]
3d1,5-(1-苄基-3,4-二甲基-2-氧代-5-(对甲苯基)-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),58%产率(65mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.19
–
7.12(m,5h),6.97(d,j=8.1hz,2h),6.90(dd,j=6.6,3.0hz,2h),4.58
–
4.49(m,2h),2.38(s,3h),2.25(qt,j=16.9,7.1hz,2h),1.81(ddd,j=13.4,11.7,5.0hz,1h),1.64
–
1.51(m,6h),1.25(s,3h),1.23
–
1.05(m,2h).
13
c{1h}nmr(101mhz,cdcl3)δ182.4,138.5,137.8,137.0,129.8,129.1,128.2,127.7,127.3,127.0,119.5,118.8,51.2,44.1,35.1,25.4,23.8,22.3,21.3,17.0,8.9.hrms(esi-tof)calcd for c
25h29
n2o
([m h]
)373.2274.found 373.2277.
[0106][0107]
3d2,5-(1-苄基-5-(4-乙基苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),71%产率(83mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.18
–
7.13(m,5h),7.00(d,j=8.1hz,2h),6.89
–
6.87(m,2h),4.58
–
4.50(m,2h),2.67(q,j=7.6hz,2h),2.25(qt,j=16.9,7.1hz,2h),1.81(ddd,j=13.3,11.5,5.1hz,1h),1.67
–
1.48(m,6h),1.30
–
1.24(m,6h),1.24
–
1.06(m,2h).
13
c{1h}nmr(101mhz,cdcl3)δ182.4,144.8,137.8,137.0,129.8,128.2,127.8,127.7,127.5,127.0,119.5,118.8,51.2,44.1,35.1,28.7,25.4,23.8,22.3,17.0,15.5,8.9.hrms(esi-tof)calcd for c
26h31
n2o
([m h]
)387.2431.found 387.2449.
[0108][0109]
3d3,5-(1-苄基-5-(4-甲氧基苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),76%产率(89mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.20
–
7.15(m,3h),7.02
–
6.98(m,2h),6.90(dd,j=6.9,2.7hz,2h),
6.88
–
6.84(m,2h),4.57
–
4.48(m,2h),3.83(s,3h),2.25(qt,j=16.9,7.1hz,2h),1.81(ddd,j=13.3,11.4,5.1hz,1h),1.64
–
1.52(m,6h),1.29
–
1.07(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ182.4,159.7,137.9,136.7,131.2,128.2,127.6,127.0,122.5,119.5,118.8,113.8,55.3,51.2,44.1,35.1,25.4,23.8,22.3,17.0,8.9.hrms(esi-tof)calcd for c
25h29
n2o
2
([m h]
)389.2224.found 389.2228.
[0110][0111]
3d4,5-(1-苄基-5-(4-(苄氧基)苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),63%产率(87mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.46
–
7.32(m,5h),7.17
–
7.14(m,3h),6.99(d,j=8.8hz,2h),6.93(d,j=8.8hz,2h),6.88(dd,j=6.7,3.0hz,2h),5.08(s,2h),4.57
–
4.48(m,2h),2.25(qt,j=16.9,7.1hz,2h),1.81(ddd,j=13.2,11.4,5.1hz,1h),1.66
–
1.52(m,6h),1.25(s,3h),1.23
–
1.06(m,2h).
13
c{1h}nmr(101mhz,cdcl3)δ182.4,158.9,137.9,136.7,136.6,131.2,128.6,128.2,128.1,127.6,127.5,127.0,122.8,119.5,118.8,114.7,70.0,51.2,44.1,35.1,25.4,23.8,22.3,17.0,8.9.hrms(esi-tof)calcd for c
31h33
n2o
2
([m h]
)465.2537.found 465.2539.
[0112][0113]
3d5,5-(1-苄基-5-(4-溴苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),60%产率(79mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.48
–
7.45(m,2h),7.19
–
7.15(m,3h),6.95
–
6.92(m,2h),6.89
–
6.86(m,2h),4.58
–
4.48(m,2h),2.27(qt,j=16.9,7.0hz,2h),1.83(ddd,j=13.3,11.4,5.3hz,1h),1.67
–
1.51(m,6h),1.26(s,3h),1.25
–
1.10(m,2h).
13
c{1h}nmr(101mhz,cdcl3)δ182.2,137.5,135.9,131.6,131.4,129.3,128.3,127.5,127.2,122.8,119.9,119.5,51.3,44.2,35.1,25.4,23.8,22.3,17.0,8.8.hrms(esi-tof)calcd for c
24h26
brn2o
([m h]
)437.1223.found 437.1222.
[0114][0115]
3d6,5-(1-苄基-5-(4-氯苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),75%产率(88mg),无色油状物.1h nmr
(400mhz,cdcl3)δ7.32
–
7.29(m,2h),7.19
–
7.15(m,3h),7.01
–
6.98(m,2h),6.89
–
6.86(m,2h),4.58
–
4.48(m,2h),2.27(qt,j=17.0,7.1hz,2h),1.83(ddd,j=13.4,11.4,5.3hz,1h),1.65
–
1.54(m,6h),1.26(s,3h),1.25
–
1.10(m,2h).
13
c{1h}nmr(101mhz,cdcl3)δ182.2,137.5,135.9,134.6,131.2,128.8,128.6,128.3,127.5,127.2,119.9,119.5,51.3,44.2,35.1,25.4,23.8,22.3,17.0,8.8.hrms(esi-tof)calcd for c
24h26
cln2o
([m h]
)393.1728.found 393.1728.
[0116][0117]
3d7,5-(1-苄基-5-(4-氟苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),71%产率(81mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.20
–
7.14(m,3h),7.05
–
6.99(m,4h),6.89
–
6.84(m,2h),4.58
–
4.48(m,2h),2.28(qt,j=16.9,7.1hz,2h),1.84(ddd,j=13.3,11.4,5.2hz,1h),1.64
–
1.54(m,6h),1.31
–
1.10(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ182.2,162.7(d,j=248.7hz),137.6,136.0,131.8(d,j=8.2hz),128.3,127.5,127.2,126.3(d,j=3.5hz),119.6,119.5,115.5(d,j=21.6hz),51.2,44.1,35.1,25.4,23.8,22.3,17.0,8.8.
19
f nmr(376mhz,cdcl3)δ-112.24(p,j=7.1hz,1f).hrms(esi-tof)calcd for c
24h26
fn2o
([m h]
)377.2024.found 377.2020.
[0118][0119]
3d8,5-(1-苄基-3,4-二甲基-2-氧代-5-(4-(三氟甲基)苯基)-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),74%产率(95mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.59(d,j=8.0hz,2h),7.20
–
7.15(m,5h),6.85
–
6.82(m,2h),4.61
–
4.50(m,2h),2.37
–
2.21(m,2h),1.86(ddd,j=13.3,11.2,5.4hz,1h),1.67
–
1.55(m,6h),1.30
–
1.15(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ182.2,137.3,135.7,134.1(d,j=1.1hz),130.6(d,j=32.8hz),130.2,128.4,127.5,127.3,125.3(q,j=3.7hz),123.9(d,j=272.3hz),120.6,119.4,51.4,44.3,35.1,25.3,23.8,22.3,17.0,8.8.
19
fnmr(376mhz,cdcl3)δ-62.75(s,3f).hrms(esi-tof)calcd for c
25h26
f3n2o
([m h]
)427.1992.found 427.2007.
[0120]
[0121]
3d9,5-(5-([1,1'-连苯基]-4-基)-1-苄基-4-乙基-3-甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),50%产率(67mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.63
–
7.60(m,2h),7.58
–
7.55(m,2h),7.49
–
7.44(m,2h),7.40
–
7.36(m,1h),7.18
–
7.13(m,5h),6.91
–
6.89(m,2h),4.54(s,2h),2.35
–
1.99(m,4h),1.86(ddd,j=13.3,11.7,5.0hz,1h),1.71
–
1.54(m,3h),1.36
–
1.24(m,4h),1.21
–
1.10(m,1h),0.97(t,j=7.6hz,3h).
13
c{1h}nmr(101mhz,cdcl3)δ182.0,141.4,140.3,137.7,130.3,129.6,128.9,128.2,127.71,127.69,127.1,127.05,126.95,124.8,119.5,51.8,44.1,35.9,25.5,23.9,23.2,17.7,17.1,15.1.hrms(esi-tof)calcd for c
31h33
n2o
([m h]
)449.2587.found 449.2587.
[0122][0123]
3d10,5-(1-苄基-5-(3-氯苯基)-3,4-二甲基-2-氧代-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),63%产率(74mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.34(ddd,j=8.1,2.1,1.2hz,1h),7.27(dd,j=7.8,7.8hz,1h),7.17(ddd,j=6.6,2.9,2.9hz,3h),7.01(dd,j=1.8,1.8hz,1h),6.95(ddd,j=7.5,1.4,1.4hz,1h),6.86(dd,j=6.6,2.9hz,2h),4.59
–
4.49(m,2h),2.36
–
2.21(m,2h),1.84(ddd,j=13.3,11.3,5.4hz,1h),1.67
–
1.54(m,6h),1.27(s,3h),1.25
–
1.10(m,2h).
13
c{1h}nmr(101mhz,cdcl3)δ182.2,137.5,135.7,134.3,132.1,129.9,129.6,128.8,128.3,128.1,127.6,127.3,120.1,119.5,51.3,44.3,35.0,25.4,23.8,22.3,17.0,8.8.hrms(esi-tof)calcd for c
24h26
cln2o
([m h]
)393.1728.found 393.1724.
[0124][0125]
3d11,5-(1-苄基-3,4-二甲基-2-氧代-5-(3-(三氟甲基)苯基)-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),62%产率(79mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.61(d,j=7.8hz,1h),7.46(dd,j=7.7,7.7hz,1h),7.27
–
7.23(m,2h),7.17
–
7.13(m,3h),6.82
–
6.79(m,2h),4.58(d,j=15.3hz,1h),4.49(d,j=15.3hz,1h),2.38
–
2.24(m,2h),1.86(ddd,j=13.3,11.0,5.7hz,1h),1.71
–
1.55(m,5h),1.33
–
1.19(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ182.1,137.3,135.6,133.2(d,j=0.7hz),131.3,130.9(q,j=32.6hz),128.9,128.4,127.4,127.3,126.6(q,j=3.7hz),125.3(q,j=3.7hz),123.7(q,j=272.6hz),120.5,119.4,51.4,44.3,35.0,25.4,23.8,22.3,17.0,8.8.
19
f nmr(376mhz,cdcl3)δ-62.75(s,3f).hrms(esi-tof)calcd for c
25h26
f3n2o
([m h]
)427.1992.found 427.1993.
major and 1h minor),7.24
–
7.06(stack,4h major and 4h minor),6.82
–
6.76(stack,2h major and 2h minor),4.86(d,j=15.0hz,1h minor),4.82(d,j=15.2hz,1h major),4.68(d,j=15.1hz,1h major),4.66(d,j=15.0hz,1h minor),2.51
–
1.85(stack,7h major and 7h minor),1.72
–
1.49(stack,5h major and 5h minor),1.36
–
1.15(stack,5h major and 5h minor).
13
c{1h}nmr(101mhz,cdcl3)δ181.3(major),180.0(minor),149.92(minor),149.87(major),149.8(major),149.4(minor),139.2(minor),138.5(major),137.5(major),137.2(minor),136.9(minor),136.7(major),128.4(minor),128.2(major),127.6(major),127.5(minor),127.4(minor),127.2(major),125.23(minor),125.20(major),123.8(minor),123.5(major),122.3(major),121.0(minor),119.4(major),119.2(minor),119.0(major),118.8(minor),51.9(major),51.1(minor),44.1(minor),44.0(major),35.7(major),31.6(minor),25.63(minor),25.57(major),25.4,23.8,23.5(minor),23.4,22.9,22.2(minor),17.05(major),17.02(minor),16.98(major),12.8(minor).hrms(esi-tof)calcd for c
26h29
n4o
([m h]
)413.2336.found 413.2338.
[0132][0133]
3e',n-苄基-n-(5-氰基-1-(吡啶-2-基)戊-1-烯-1-基)甲基丙烯酰胺,快速柱层析分离(石油醚/乙酸乙酯=14:1),28%产率(29mg),无色油状物.1h nmr(400mhz,cdcl3)δ8.67(dd,j=5.4,1.9hz,1h),7.70(ddd,j=7.8,7.8,1.9hz,1h),7.32
–
7.22(m,7h),5.34
–
5.27(m,2h),5.09(s,1h),4.70(s,2h),2.52(q,j=7.5hz,2h),2.17(t,j=7.2hz,2h),1.83(s,3h),1.66(p,j=7.3hz,2h).
13
c{1h}nmr(101mhz,cdcl3)δ172.5,154.1,149.6,141.2,139.6(br),137.3,136.4,131.7,129.0,128.4,127.5,123.2,122.8,119.5,117.6(br),50.6(br),27.1,25.0,20.4,16.4.hrms(esi-tof)calcd for c
22h24
n3o
([m h]
)346.1914.found 346.1917.
[0134][0135]
3f,5-(1-苄基-3-甲基-2-氧代-2,3,4,5,6,7-六氢-1h-吲哚-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),65%产率(63mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.33
–
7.16(m,5h),4.63(s,2h),2.34
–
2.19(m,2h),2.17
–
1.87(m,4h),1.77
–
1.41(m,8h),1.24
–
1.07(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ182.6,138.1,135.4,128.6,127.3,127.1,119.6,118.9,50.2,43.1,34.9,25.4,24.0,22.54,22.46,22.2,21.4,19.8,17.0.hrms(esi-tof)calcd for c
21h27
n2o
([m h]
)323.2118.found 323.2122.
基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=15:1),42%产率(42mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.46
–
7.37(m,3h),7.30
–
7.27(m,2h),3.63(dt,j=14.2,6.1hz,1h),3.49(dt,j=14.2,5.9hz,1h),3.24(qt,j=10.1,6.0hz,2h),3.16(s,3h),2.40
–
2.25(m,2h),1.79(ddd,j=13.3,11.1,5.5hz,1h),1.72
–
1.52(m,6h),1.32
–
1.17(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ182.6,136.9,130.5,129.8,128.6,128.5,119.5,118.8,69.6,58.4,51.2,39.7,35.0,25.4,23.6,22.2,17.0,8.9.hrms(esi-tof)calcd for c
20h27
n2o
2
([m h]
)327.2067.found 327.2069.
[0144][0145]
3i,5-(3,4-二甲基-1-(2-吗啉基乙基)-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),52%产率(60mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.46
–
7.38(m,3h),7.29
–
7.27(m,2h),3.57
–
3.45(m,6h),2.40
–
2.16(m,8h),1.78(ddd,j=13.3,10.2,6.4hz,1h),1.72
–
1.52(m,6h),1.33
–
1.20(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ182.5,136.7,130.6,129.6,128.64,128.59,119.5,119.0,66.9,56.7,53.4,51.2,37.2,34.9,25.4,23.6,22.4,17.0,8.8.hrms(esi-tof)calcd for c
23h32
n3o
2
([m h]
)382.2489.found 382.2501.
[0146][0147]
3j1,5-(4-乙基-1-(3-甲氧基苄基)-3-甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),63%产率(76mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.39
–
7.30(m,3h),7.12
–
7.08(m,2h),7.06(d,j=7.9hz,1h),6.70(ddd,j=8.3,2.7,1.0hz,1h),6.47(ddd,j=7.6,1.7,0.9hz,1h),6.38(dd,j=2.6,1.6hz,1h),4.47(s,2h),3.68(s,3h),2.35
–
2.18(m,2h),2.04(ddt,j=32.9,14.7,7.5hz,2h),1.85(ddd,j=13.3,11.7,5.0hz,1h),1.69
–
1.52(m,3h),1.35
–
1.23(s,4h),1.22
–
1.10(m,1h),0.93(t,j=7.6hz,3h).
13
c{1h}nmr(101mhz,cdcl3)δ182.0,159.4,139.3,137.9,130.7,130.0,129.2,128.7,128.3,124.5,120.0,119.6,113.0,112.9,55.1,51.8,44.0,35.8,25.5,23.9,23.2,17.6,17.0,15.0.hrms(esi-tof)calcd for c
26h31
n2o
2
([m h]
)403.2380.found 403.2383.
[0148][0149]
3j2,5-(3,4-二甲基-1-(4-甲基苄基)-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)
戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),61%产率(66mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.37
–
7.33(m,3h),7.10
–
7.08(m,2h),6.95(d,j=7.8hz,2h),6.75(d,j=8.0hz,2h),4.50(s,2h),2.34
–
2.17(m,5h),1.82(ddd,j=13.3,11.5,5.1hz,1h),1.65
–
1.53(m,6h),1.29
–
1.08(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ182.4,137.0,136.6,134.7,130.4,129.9,128.9,128.5,128.3,127.6,119.5,119.0,51.2,43.9,35.1,25.4,23.8,22.3,21.1,17.0,8.8.hrms(esi-tof)calcd for c
25h29
n2o
([m h]
)373.2274.found 373.2275.
[0150][0151]
3j3,5-(1-(4-氯苄基)-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),75%产率(89mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.38
–
7.33(m,3h),7.14
–
7.11(m,2h),7.10
–
7.07(m,2h),6.80
–
6.77(m,2h),4.56
–
4.46(m,2h),2.36
–
2.21(m,2h),1.82(ddd,j=13.3,11.6,5.0hz,1h),1.67
–
1.54(m,6h),1.28
–
1.07(m,5h).
13
c{1h}nmr(101mhz,cdcl3)δ182.4,136.7,136.2,132.9,130.2,129.8,129.1,128.7,128.5,128.4,119.5,119.3,51.2,43.5,35.1,25.4,23.8,22.3,17.0,8.8.hrms(esi-tof)calcd for c
24h26
cln2o
([m h]
)393.1728.found 393.1726.
[0152][0153]
3j4,5-(1-(4-氟苄基)-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),61%产率(69mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.40
–
7.33(m,3h),7.10
–
7.06(m,2h),6.86
–
6.78(m,4h),4.56
–
4.47(m,2h),2.36
–
2.19(m,2h),1.83(ddd,j=13.3,11.6,5.1hz,1h),1.66
–
1.54(m,6h),1.25(s,3h),1.24
–
1.07(m,2h).
13
c{1h}nmr(101mhz,cdcl3)δ182.4,161.9(d,j=245.4hz),136.7,133.5(d,j=3.3hz),130.3,129.8,129.4(d,j=8.1hz),128.7,128.4,119.5,119.3,115.1(d,j=21.3hz),51.2,43.4,35.1,25.4,23.8,22.3,17.0,8.8.
19
f nmr(376mhz,cdcl3)δ-115.40(p,j=7.1,6.4hz,1f).hrms(esi-tof)calcd for c
24h26
fn2o
([m h]
)377.2024.found 377.2025.
[0154][0155]
3k,5-(1-烯丙基-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)戊腈,快速柱层析分离(石油醚/乙酸乙酯=21:1),36%产率(38mg),无色油状物.1h nmr(400mhz,
minor),4.55
–
4.46(stack,2h major and 2h minor),3.76(s,3h minor),3.75(s,3h major),2.72
–
2.48(stack,3h major and 3h minor),1.87
–
1.76(stack,1h major and 1h minor),1.63
–
1.27(stack,6h major and 6h minor),1.26(s,3h major),1.25(s,3h minor).
13
c{1h}nmr(101mhz,cdcl3)δ181.93(minor),181.88(major),172.7(minor),172.5(major),137.0(major),136.9(minor),136.20(minor),136.18(major),133.0(major and minor),130.1(minor),130.0(major),129.8(major and minor),129.2(major),129.1(minor),128.80(major),128.77(minor),128.5,128.48(minor),128.39(major),118.9(minor),118.8(major),117.5(major),117.3(minor),52.47(major),52.45(minor),51.0(minor),50.9(major),43.51(minor),43.49(major),41.2(minor),41.1(major),32.7(minor),32.4(major),26.3(minor),26.0(major),22.19(major),22.16(minor),19.7(minor),18.8(major),8.71(major),8.67(minor).hrms(esi-tof)calcd for c
26h28
cln2o
3
([m h]
)451.1783.found 451.1784.
[0162][0163]
3o,2-(2-(1-(4-氯苄基)-3,4-二甲基-2-氧代-5-苯基-2,3-二氢-1h-吡咯-3-基)乙基)-2-(氰甲基)丙二酸二异丙酯,快速柱层析分离(石油醚/乙酸乙酯=21:1),57%产率(97mg),无色油状物.1h nmr(400mhz,cdcl3)δ7.39
–
7.32(m,3h),7.14
–
7.11(m,2h),7.10
–
7.07(m,2h),6.82
–
6.79(m,2h),5.10(heptd,j=6.3,2.1hz,2h),4.58(d,j=15.3hz,1h),4.45(d,j=15.3hz,1h),2.97
–
2.88(m,2h),1.92
–
1.84(m,1h),1.70(qd,j=13.0,3.7hz,2h),1.63(s,3h),1.55(td,j=13.0,4.3hz,1h),1.27(td,j=6.2,3.1hz,15h).
13
c{1h}nmr(101mhz,cdcl3)δ181.6,168.1,168.0,137.1,136.3,132.8,130.1,129.9,129.1,128.7,128.4,118.6,116.2,70.5,70.3,54.7,50.8,43.5,30.3,27.8,22.1,21.9,21.5,21.4,8.7.hrms(esi-tof)calcd for c
32h38
cln2o
5
([m h]
)565.2464.found 565.2466。
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。
