1.本发明属于医药领域,特别涉及一类木豆素衍生物及其在制备抗菌药物中的应用。
背景技术:
2.耐药菌株的进化是通过抗生素对微生物种群的选择压力而产生的一种自然现象,抗菌药物的误用及滥用加速了细菌的耐药进程(chellat m f,raguz l,riedl r.angew.chem.int.ed.2016,55:6600
–
6626)。抗生素耐药性及其全球传播不仅威胁抗生素自身的持续有效性,而且还可能危及全球卫生安全。目前,抗生素的研发周期长,细菌产生耐药的进化速度领先于新药上市的速度,打击了传染病控制的核心,有可能阻止甚至逆转药物的治疗进展(sharma a.indian j.med.microbiol.2011,29(2):91)。为了应对这一正在出现的危机,世界卫生组织等全球组织敦促科学界寻找对抗抗生素耐药性的新方法。
3.20世纪60年代初,随着甲氧西林的发现和广泛的临床应用,金黄色葡萄球菌很快对这种药物产生了耐药性,即产生耐甲氧西林金黄色葡萄球菌(mrsa)。最初,mrsa的传播发生在临床环境中,但最近几十年,它的存在已被报道在社区,一些医院环境之外的地方(oniciuc ea,ariza-miguel j,bolocan as et al.int j food microbiol.2014,209:34
–
38)。与不耐药菌株相比,mrsa引起的感染在公共卫生领域产生了更高的费用,其发病率和死亡率也更高(cosgrove s,sakoulas g,perencevich e,schwaber m,karchmer a,carmeli y.clin infect dis.36(1):53
–
59),在美国,每年死于mrsa感染的人比死于hiv的人还多(farias aln.rev med.2015,55(2):39
–
45)。目前,万古霉素被用作对抗mrsa的最后一种抗生素,然而,万古霉素使用量增加所带来的高选择性压力促进了耐万古霉素的金黄色葡萄球菌的出现(chambers hf,deleo fr.nat.rev.microbiol.2009,7(9),629
–
641.weigel lm,clewell db,gill sr et al.2003,science 302(5650):1569
–
1571)。
4.细菌进入休眠模式也是应对抗生素的生存策略,如持留菌的出现,与传统的细菌耐药相比,持留菌表现的是对抗生素耐受,通过降低代谢活动、增加外排泵的表达,从而减少药物的吸收;藏身于生物膜、宿主细胞内,躲避抗生素、免疫系统的攻击。持留菌是慢性感染及复发性感染的重要病因,也是耐药细菌产生的源泉(zhang,ying.emerg.microbes infect.2014,3(1):1-8.cohen n r,lobritz m a,collins j j.cell host microbe,2013,13(6):632-642.fisher r a,gollan b,helaine s.nat.rev.microbiol,2017,15:453-464)。目前临床上尚无有效药物可以清除持留菌。因此,研发新型抗菌剂,尤其是针对耐药菌以及持留菌的药物具有重大的意义。
技术实现要素:
5.为了克服上述现有技术的缺点与不足,本发明的首要目的在于提供一类以细菌细胞膜为靶标的木豆素结构衍生物。
6.本发明另一目的在于提供上述木豆素结构衍生物的制备方法。
7.本发明再一目的在于提供上述木豆素结构衍生物在制备抗菌药物中的应用,尤其在制备体内外抗mrsa持留菌药物以及清除mrsa生物膜药物中的应用。
8.本发明的目的通过下述方案实现:
9.一类木豆素结构类似化合物,具有如式ⅰ所示的化学结构:
[0010][0011]
其中,r1为取代苯环;r2为乙基、环丙甲基、含异丙基结构单元的支链烷烃、末端以1-3个氟原子取代的含2-4个碳原子的直链烷基、含有4-7个碳原子的环状烷烃、苄基或者四氢吡喃环。
[0012]
所述的r1优选为4-氟苯基、4-三氟苯基中的一种。
[0013]
优选的,所述的木豆素结构衍生物,具有如下所示的化学结构:
[0014]
[0015][0016]
一种上述木豆素结构衍生物的制备方法,具体包括以下步骤:
[0017]
(1.1)将2,4,6-三羟基苯甲酸、dmap(4-二甲氨基吡啶)、无水丙酮和氯化亚砜在乙二醇二甲醚中进行反应,反应结束后得到的反应液经纯化即得化合物1;
[0018]
(1.2)将化合物1、三苯基膦、diad(偶氮二甲酸二异丙酯)及苯甲醇置于无水四氢呋喃中反应,得到的反应液经纯化所得到化合物2;
[0019]
(1.3)将所得的化合物2与三氟甲磺酸酐在吡啶中反应,反应结束后得到的反应液经纯化所得到化合物3;
[0020]
(1.4)化合物3与取代苯乙烯(ch2=ch-r1)、双三苯基膦合二氯化钯在无水dmf/tea混合溶剂中加热封管反应,得到的反应液经纯化得到化合物4;
[0021]
(1.5)化合物4与碳酸钾在甲醇中加热反应,得到的反应液经纯化所得的化合物为化合物5;
[0022]
(1.6)化合物5与三氯化硼在无水二氯甲烷中进行反应,所得的反应液经纯化后得到化合物6;
[0023]
(1.7)化合物6与卤代脂肪烃(r2x)、碳酸钾在无水dmf中加热反应,得到的反应液经纯化即为化合物7;或者将化合物6与脂肪醇(r2oh)、diad及三苯基膦在无水四氢呋喃反应,得到的反应液经纯化即为化合物7;
[0024]
(1.8)冰浴条件下,化合物7与氢化钠在干燥甲苯搅拌反应,然后加入1-溴-3-甲基-2-丁烯,加热继续反应,所得的反应液经纯化即为化合物8;
[0025]
(1.9)将化合物8溶解于乙醇中,加入氢氧化钠水溶液,加热搅拌,反应液经纯化即得式ⅰ所示木豆素结构衍生物;
[0026]
此时的合成路线为:
[0027][0028]
步骤(1.1)中所述的2,4,6-三羟基苯甲酸、丙酮、二氯亚砜、dmap的摩尔比为1:1~2:2~3:0.05~0.1;步骤(1.1)中所述的反应是指在0~30℃反应4~8h;
[0029]
步骤(1.2)中所述的化合物1与苯甲醇、三苯基膦、diad的摩尔比为1:1~1.5:1~1.5:1~1.5;步骤(1.2)中所述的反应时间为2~12h,反应温度指0~30℃;步骤(1.2)中所述的纯化方法是指将反应液加进冰镇的石油醚中,析出固体,减压过滤,收集滤液,减压浓缩,滤渣经柱层析纯化即得化合物3;
[0030]
步骤(1.3)中所述的化合物2、三氟甲磺酸酐的摩尔比为1:1~2;步骤(1.3)所述的反应是指在0~30℃反应1~8h;步骤(1.3)所述的纯化手段是指将反应体系置于冰水浴,以饱和氯化铵溶液淬灭,以2mol/l hcl将反应液的ph调整为2~3,以乙酸乙酯萃取,合并有机相,并以饱和氯化钠溶液洗涤,以无水硫酸钠干燥,过滤,减压浓缩滤液,经柱层析纯化得化合物3;
[0031]
步骤(1.4)中所述的取代苯乙烯优选为4-氟苯乙烯或4-三氟甲基苯乙烯;步骤(1.4)中所述的化合物3、双三苯基膦合二氯化钯、取代苯乙烯的摩尔比为1:0.05~0.5:1~2;步骤(1.4)所述的dmf和tea体积之比为5:1;步骤(1.4)所述的反应是置于封管中进行,反应温度为100~150℃,反应时间为8h;步骤(1.4)所述的纯化方法为将反应装置静置,降温至室温,加水淬灭,以乙酸乙酯萃取,萃取得到的有机物经柱层析手段纯化得到化合物4;
[0032]
步骤(1.5)所述的化合物4、碳酸钾的摩尔比为1:1.1~1.5;步骤(1.5)所述的加热反应温度为25~50℃;步骤(1.5)所述的加热反应的时间为1~12h;步骤(1.5)所述的纯化步骤为减压除去甲醇,向残渣加水,以2mol/l hcl调节ph为2~3,以乙酸乙酯萃取,饱和氯化钠洗涤,无水硫酸钠干燥,收集滤液并除去溶剂,柱层析纯化得化合物5;
[0033]
步骤(1.6)所述的化合物5、三氯化硼的摩尔比为1:1.1~2.5;步骤(1.6)所述的反应是在0~30℃反应1~4h;步骤(1.6)所述的纯化步骤为冰水淬灭反应,以二氯甲烷萃取,收集有机相,并以无水硫酸钠干燥,过滤并将溶剂蒸除,残渣通过柱层析纯化得到化合物6;
[0034]
步骤(1.7)所述的卤代脂肪烃r2x优选为2-碘-1,1,1-三氟乙烷或1-溴-4-氟丁烷;步骤(1.7)所述的化合物6、卤代脂肪烃r2x、碳酸钾的摩尔比为1:2~3:2~3;加入卤代脂肪烃r2x的步骤(1.7)中所述的加热反应是指在80~100℃条件下反应3~24h,反应结束后将
反应体系冷却至室温,以水淬灭反应,乙酸乙酯萃取,有机相以饱和氯化钠洗涤,以无水硫酸钠干燥,过滤,减压蒸除滤液溶剂,所得残余物以硅胶柱层析纯化得到化合物7;
[0035]
步骤(1.7)所述的脂肪醇r2oh优选为乙醇、异丙醇、仲丁醇、3-戊醇、3-己醇、环丙甲醇、环丁醇、环戊醇、环己醇、环庚醇、苯甲醇、四氢吡喃-4-醇、2-氟乙醇、2,2-二氟乙醇、3-氟丙醇、3,3,3-三氟丙-1-醇或4,4,4-三氟丁醇;步骤(1.7)所述的化合物7、脂肪醇r2oh、三苯基膦、diad的摩尔比为1:1~1.5:1~1.5:1~1.5;步骤(1.7)所述的在四氢呋喃中反应是在0~30℃反应2~12h,反应结束后将反应液加入到冰镇的石油醚中,析出固体,减压过滤,收集滤液,减压浓缩,滤渣经柱层析纯化即得化合物7;
[0036]
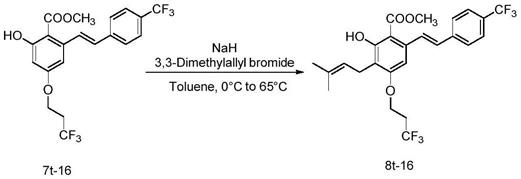
步骤(1.8)所述的化合物7、氢化钠、1-溴-3-甲基-2-丁烯的摩尔比为1:1.3~2:1.3~2;步骤(1.8)所述的搅拌反应是在冰水浴条件下搅拌20~30min;步骤(1.8)所述的加热继续反应是指加热至65℃反应2~4h;步骤(1.8)所述的纯化方法如下:反应装置放冷至室温,加饱和氯化铵溶液淬灭反应,以乙酸乙酯萃取,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤收集滤液,蒸除溶剂,残余物经硅胶柱层析纯化得到化合物8;
[0037]
步骤(1.9)所述的化合物8、氢氧化钠摩尔比为1:5~10;步骤(1.9)所述的加热搅拌是指在80~100℃搅拌2-12h;步骤(1.9)所述的纯化方法如下:将反应体系放冷至室温,以2mol/l hcl调节ph 2~3,待固体析出,过滤,收集的滤渣经重结晶即得木豆素结构衍生物。
[0038]
上述的木豆素结构衍生物在制备抗菌药物中的应用。尤其是在制备抗耐甲氧西林的金黄色葡萄球菌、抗耐甲氧西林的金黄色葡萄球菌持留菌药物中的应用。
[0039]
所述的药物指包含木豆素结构衍生物或其药用盐和溶剂化物中的至少一种。
[0040]
所述的药物还包含一种或多种药学上可接受的载体或赋形剂。
[0041]
本发明的机理为:
[0042]
本发明以一个抗菌天然产物作为先导化合物,以细菌细胞膜为靶点,通过引入大位阻的疏水基团、含氟侧链及含氮亲水基团设计了一系列化合物。采用实际可行的合成方法与适当的试剂设计了目标化合物的合成路线。
[0043]
本发明相对于现有技术,具有如下的优点及有益效果:
[0044]
本发明所采用的合成路线简单,使用原料皆商业可得。本发明合成的木豆素类衍生物较木豆素而言具有更高抗菌活性。
附图说明
[0045]
图1为实施例中10μg/ml的化合物对两种哺乳动物细胞(小鼠巨噬细胞raw264.7和正常人肝细胞lo2)的mtt毒性实验图;
[0046]
图2为实施例中清除持留菌初筛结果图;
[0047]
图3为实施例中不同浓度化合物y8清除持留菌的实验结果图。
[0048]
图4为实施例中不同浓度化合物y8清除mrsa成熟生物膜的实验结果图。
[0049]
图5为实施例中不同浓度化合物y8清除体内持留菌的菌落计数结果图。
[0050]
图6为实施例中不同浓度化合物y8清除体内持留菌的小鼠内脏he染色图。
具体实施方式
[0051]
下面结合实施例和附图对本发明作进一步详细的描述,但本发明的实施方式不限于此。
[0052]
实施例中所用试剂如无特殊说明均可从市场常规购得。
[0053]
实施例1:5,7-二羟基-2,2-二甲基-4h-苯并[d][1,3]二氧杂环己烯-4-酮(1)的制备
[0054][0055]
向一个装有磁力搅拌子的250ml干燥双颈瓶加入商业可得的起始原料2,4,6-三羟基苯甲酸(10g,58.78mmol),dmap(431mg,3.53mmol),丙酮(6.5ml,88.17mmol)以及100ml乙二醇二甲醚,反应体系置于冰水浴中搅拌,直至固体溶解。向反应体系中逐滴加入氯化亚砜(8.5ml,117.56mmol),滴加完毕后逐渐升温至室温。8h后,取1000ml的圆底烧瓶,加入300ml饱和碳酸氢钠溶液,置于冰水浴下冷却,将反应液缓慢加入冰镇的饱和碳酸氢钠溶液中,直至气泡消失,以乙酸乙酯萃取,饱和氯化钠溶液洗涤,无水硫酸钠干燥,减压除去溶剂,残渣经硅胶柱层析纯化(石油醚:丙酮=10:1),得白色固体9.8g,收率为80%;1h nmr(400mhz,dmso-d6)δ10.84(s,1h),10.29(s,1h),6.01(d,j=2.1hz,1h),5.93(d,j=2.1hz,1h),1.65(s,6h).
13
c nmr(101mhz,dmso)δ166.5,163.8,162.3,156.9,106.5,97.4,95.4,91.8,25.1;esi-lrms m/z:208.9[m-h]-.
[0056]
实施例2:7-苄氧基-5-羟基-2,2-二甲基-4h-苯并[d][1,3]二氧杂环己烯-4-酮(2)的制备
[0057][0058]
将化合物1(9.7g,46.15mmol),三苯基膦(13.3g,50.77mmol),苯甲醇(5.3ml,50.77mmol)置于干燥的250ml双颈烧瓶中,加入100ml无水thf使固体充分溶解,冰水浴环境下搅拌20分钟后,缓慢滴加diad(10.0ml,50.77mmol),滴加完毕,逐渐升温至室温,tlc检测反应结束,将反应液加进300ml的冰镇石油醚中,待白色固体充分析出,过滤,收集滤液,减压蒸除溶剂,残渣以硅胶柱层析纯化(石油醚:乙酸乙酯=40:1),得到化合物2为白色固体9.9g,收率为72%;1h nmr(300mhz,cdcl3)δ10.50(s,1h),7.47
–
7.25(m,5h),6.23(d,j=2.3hz,1h),6.10(d,j=2.3hz,1h),5.03(s,2h),1.71(s,6h).
13
c nmr(75mhz,cdcl3)δ166.6,165.0,162.9,156.8,135.5,128.6,128.3,127.4,106.8,96.5,95.2,93.1,70.3,25.5;esi-lrms m/z:298.9[m-h]-.
[0059]
实施例3:7-苄氧基-2,2-二甲基-4-氧代-4h-苯并[d][1,3]二氧杂环己烯-5-基三氟甲磺酸酯(3)的制备
[0060][0061]
化合物2(9.7g,32.31mmol)以40ml吡啶溶解于100ml的支口烧瓶中,置于冰水浴环境,氮气保护下,缓慢加入三氟甲磺酸酐(7.1ml,42.01mmol),1h后,以饱和氯化铵溶液淬灭反应,冰水浴条件下,缓慢加入10%hcl溶液50ml,水相以乙酸乙酯萃取,收集有机相,并以饱和氯化钠洗涤,无水硫酸钠干燥,过滤所得的滤液的溶剂经减压除去,所得残余物经硅胶柱层析纯化(石油醚:乙酸乙酯=20:1),得到化合物3黄色油状物13.3g,收率为95%;1h nmr(300mhz,cdcl3)δ7.48
–
7.33(m,5h),6.61(d,j=2.3hz,1h),6.60(d,j=2.3hz,1h),5.11(s,2h),1.72(s,6h).
13
c nmr(75mhz,cdcl3)δ164.7,158.8,157.2,149.8,134.7,128.8,127.7,118.7(q,1j=318.8hz),106.7,105.9,102.1,101.0,71.2,25.4;esi-lrms m/z:430.7[m-h]-.
[0062]
实施例4:(e)-7-苄氧基-5-(4-氟苯乙烯基)-2,2-二甲基-4h-苯并[d][1,3]二氧杂环己烯-4-酮(4y)的制备
[0063][0064]
取dmf/tea混合溶液(体积比为5/1,共50ml)将化合物3(13.1g,30.32mmol)、双三苯基膦合二氯化钯(1.1g,1.52mmol)及4-氟苯乙烯(4.6ml,45.48mmol)转移至带磁力搅拌子的100ml干燥封管中,通氮气,超声鼓泡20分钟,密封,置于120℃搅拌反应。8-12小时后,将反应装置放冷至室温,加水淬灭反应,加乙酸乙酯萃取,有机相以饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤后所得滤液经减压浓缩得到残渣,残渣以硅胶柱层析纯化(石油醚:乙酸乙酯=30:1),得到化合物4y为白色固体8.7g,收率为70%;1h nmr(400mhz,cdcl3)δ8.18(d,j=16.2hz,1h),7.57
–
7.49(m,2h),7.48
–
7.37(m,5h),7.05(t,j=8.7hz,2h),7.01
–
6.96(m,2h),6.47(d,j=2.4hz,1h),5.13(s,2h),1.73(s,6h).
13
c nmr(101mhz,cdcl3)δ164.1,162.9(d,1j
c-f
=252.5hz),160.4,159.0,143.5,135.8,133.4(d,4j
c-f
=3.0hz),131.5,128.9,128.8(d,3j
c-f
=3.0hz),128.5,127.7,126.8(d,5j
c-f
=2.0hz),115.7(d,2j
c-f
=22.2hz),108.8,105.2,104.3,101.5,70.5,25.7;esi-lrms m/z:405.2[m h]
.
[0065]
实施例5:(e)-7-苄氧基-5-(4-三氟甲基苯乙烯基)-2,2-二甲基-4h-苯并[d][1,3]二氧杂环己烯-4-酮(4t)的制备
[0066][0067]
参照化合物4y的合成,以化合物3(12.3g,28.47mmol)为原料,仅将4-氟苯乙烯替换为4-三氟甲基苯乙烯,得到化合物4t为黄色固体8.9g,收率为73%;1h nmr(400mhz,
cdcl3)δ8.33(d,j=16.2hz,1h),7.62(dd,j=19.3,8.1hz,4h),7.49
–
7.32(m,5h),7.02(m,2h),6.50(d,j=2.0hz,1h),5.14(s,2h),1.73(s,6h).
13
c nmr(101mhz,cdcl3)δ164.2,160.4,159.1,142.9,140.6,135.7,131.0,129.6,129.8(q,2j
c-f
=32.3hz),128.9,128.6,127.7,127.4,125.7(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=272.7hz),109.2,105.4,104.5,102.0,70.6,25.7;esi-lrms m/z:455.2[m h]
.
[0068]
实施例6:(e)-4-(苄氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(5y)的制备
[0069][0070]
将化合物4y(8.3g,20.34mmol)置于200ml圆底烧瓶中,加50ml甲醇搅拌均匀,加入无水碳酸钾(3.1g,22.38mmol),升温至50℃。2h后反应结束,将装置放冷至室温,减压浓缩,残渣以水分散,2m hcl调节ph至中性,乙酸乙酯萃取,有机层以饱和食盐水洗涤,无水硫酸钠干燥,过滤,收集滤液,减压除去溶剂得到的残渣经硅胶柱层析纯化(石油醚:乙酸乙酯=30:1),得到化合物5y为白色固体6.5g,收率为85%;1h nmr(300mhz,cdcl3)δ11.81(s,1h),7.62(d,j=16.0hz,1h),7.52
–
7.39(m,7h),7.15
–
7.04(m,2h),6.78(d,j=16.0hz,1h),6.73(d,j=2.5hz,1h),6.55(d,j=2.5hz,1h),5.07(s,2h),3.94(s,3h).
13
c nmr(75mhz,cdcl3)δ171.5,165.1,163.2,162.4(d,1j
c-f
=246.0hz),142.6,136.0,133.6(d,4j
c-f
=3.0hz),129.6,129.5(d,5j
c-f
=3.0hz),128.6,128.1(d,3j
c-f
=9.0hz),127.6,115.6(d,2j
c-f
=21.8hz),108.4,104.0,101.1,70.0,52.2;esi-lrms m/z:376.8[m-h]-.
[0071]
实施例7:(e)-4-(苄氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(5t)的制备
[0072][0073]
参照化合物5y的合成,以化合物4t(8.7g,19.16mmol)为原料得到化合物5t,白色固体7.2g,收率为88%;1h nmr(400mhz,cdcl3)δ11.67(s,1h),7.78(d,j=15.9hz,1h),7.60(dd,j=23.5,8.2hz,4h),7.49
–
7.32(m,5h),6.80(d,j=15.9hz,1h),6.72(d,j=2.2hz,1h),6.55(d,j=2.2hz,1h),5.11(s,2h),3.95(s,3h).
13
c nmr(101mhz,cdcl3)δ171.5,165.3,163.4,142.3,141.0,136.1,132.5,129.6(q,2j
c-f
=32.3hz),129.5,128.8,128.4,127.7,126.9,125.8(q,3j
c-f
=4.0hz),124.7(q,1j
c-f
=273.7hz),109.0,104.3,101.6,70.3,52.4;esi-lrms m/z:426.8[m-h]-.
[0074]
实施例8:(e)-2-(4-氟苯乙烯基)-4,6-二羟基苯甲酸甲酯(6y)的制备
[0075][0076]
冰水浴环境下,化合物5y(6.3g,16.67mmol)以30ml无水二氯甲烷溶解在干燥的
100ml支口烧瓶中,并以氮气保护,向反应装置中缓慢滴加三氯化硼的二氯甲烷溶液(25ml,25.00mmol,1mol/l),缓慢升温至室温。tlc监测反应进程,待反应结束,以冰水淬灭反应,二氯甲烷萃取,有机层依次以水、饱和食盐水洗涤,无水硫酸钠干燥,过滤,收集滤液,经减压浓缩及硅胶柱层析纯化(石油醚:乙酸乙酯=10:1),得到白色固体3.9g,收率为82%;1h nmr(400mhz,dmso-d6)δ10.30(s,1h),9.98(s,1h),7.66
–
7.50(m,2h),7.22-7.18(m,2h),7.19(d,j=16.2hz,1h),6.98(d,j=16.2hz,1h),6.60(d,j=2.2hz,1h),6.31(d,j=2.2hz,1h),3.81(s,3h).
13
c nmr(101mhz,dmso-d6)δ169.1,161.8(d,1j
c-f
=246.5hz)160.4,159.0,138.8,133.4(d,4j
c-f
=3.0hz),129.1,128.5(d,3j
c-f
=8.1hz),126.9(d,5j
c-f
=2.0hz),115.6(d,2j
c-f
=21.2hz),109.2,104.8,102.2,52.0;esi-lrms m/z:286.8.[m-h]-.
[0077]
实施例9:(e)-2,4-二羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(6t)的制备
[0078][0079]
参照化合物6y的合成,以化合物5b(7.1g,16.57mmol)为原料得到化合物6t,黄色固体4.3g,收率为78%;1h nmr(400mhz,dmso-d6)δ10.35(s,1h),10.04(s,1h),7.73(q,j=8.5hz,4h),7.41(d,j=16.2hz,1h),7.07(d,j=16.2hz,1h),6.64(d,j=2.1hz,1h),6.35(d,j=2.1hz,1h),3.82(s,3h).
13
c nmr(101mhz,dmso-d6)δ169.0,160.5,159.1,141.0,138.5,130.0,128.7,128.1(q,2j
c-f
=32.3hz),127.2,125.6(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=273.7hz),123.0,118.3,109.2,105.3,102.7,52.0;esi-lrms m/z:336.8[m-h]-.
[0080]
实施例10:(e)-4-乙氧基-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7c-1)的制备
[0081][0082]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为乙醇,得到化合物7c-1为白色粉末499mg,收率为76%;1h nmr(400mhz,cdcl3)δ11.65(s,1h),7.59(d,j=15.9hz,1h),7.48
–
7.36(m,2h),7.05(t,j=8.6hz,2h),6.75(d,j=15.9hz,1h),6.60(d,j=2.5hz,1h),6.41(d,j=2.5hz,1h),4.07(q,j=7.0hz,2h),3.93(s,3h),1.43(t,j=7.0hz,3h).
13
c nmr(101mhz,cdcl3)δ171.7,165.3,163.7,162.5(d,1j
c-f
=248.5hz),142.7,133.8(d,4j
c-f
=3.0hz),129.8(d,5j
c-f
=3.0hz),129.7,128.2(d,3j
c-f
=8.1hz),115.8(d,2j
c-f
=21.2hz),108.5,103.9,100.8,63.9,52.3,14.7;esi-lrms m/z:314.9[m-h]-.
[0083]
实施例11:(e)-2-(4-氟苯乙烯基)-6-羟基-4-异丙氧基苯甲酸甲酯(7c-2)的制备
[0084][0085]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为异丙醇,得到化合物7c-2为白色粉末570mg,收率为83%;1h nmr(400mhz,cdcl3)δ11.66(s,1h),7.60(d,j=15.9hz,1h),7.49
–
7.38(m,2h),7.12
–
6.98(m,2h),6.76(d,j=15.9hz,1h),6.59(d,j=2.4hz,1h),6.42(d,j=2.5hz,1h),4.84
–
4.37(m,1h),3.94(s,3h),1.37(d,j=6.1hz,6h).
13
c nmr(101mhz,cdcl3)δ171.7,165.3,162.8,162.5(d,1j
c-f
=248.5hz),142.8,133.8(d,4j
c-f
=4.0hz),129.8,129.8,129.7,128.2(d,3j
c-f
=8.1hz),115.8(d,2j
c-f
=21.2hz),109.4,103.6,101.5,70.3,52.3,22.0;esi-lrms m/z:328.9[m-h]-.
[0086]
实施例12:(e)-4-(环丙基甲氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7c-3)的制备
[0087][0088]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为环丙甲醇,得到化合物7c-3为白色粉末498mg,收率为70%;1h nmr(400mhz,cdcl3)δ11.64(s,1h),7.59(d,j=15.9hz,1h),7.50
–
7.35(m,2h),7.11
–
6.97(m,2h),6.76(d,j=15.9hz,1h),6.63(d,j=2.5hz,1h),6.40(d,j=2.5hz,1h),3.93(s,3h),3.84(d,j=7.0hz,2h),1.34
–
1.19(m,1h),0.68(q,j=5.0hz,2h),0.37(q,j=5.0hz,2h).
13
c nmr(101mhz,cdcl3)δ171.7,165.3,163.7,162.5(d,1j
c-f
=248.5hz),142.7,133.8(d,4j
c-f
=3.0hz),129.7(d,5j
c-f
=3.0hz),129.7,128.2(d,3j
c-f
=8.1hz),115.8(d,2j
c-f
=22.2hz),108.5,103.9,100.8,73.0,52.3,10.1,3.3;esi-lrms m/z:340.9[m-h]-.
[0089]
实施例13:(e)-4-(环戊氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7c-4)的制备
[0090][0091]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为环戊醇,得到化合物7c-4为白色粉末496mg,收率为67%;1h nmr(400mhz,cdcl3)δ11.65(s,1h),7.60(d,j=15.9hz,1h),7.50
–
7.40(m,2h),7.04(dd,j=8.6,1.9hz,3h),6.76(d,j=15.9hz,1h),6.58(d,j=2.5hz,1h),6.41(d,j=2.5hz,1h),4.88
–
4.71(m,1h),3.93(s,3h),2.07
–
1.58(m,8h).
13
c nmr(101mhz,cdcl3)δ171.8,165.2,163.0,162.6(d,1j
c-f
=248.5hz),142.7,133.8(d,4j
c-f
=3.0hz),129.9(d,5j
c-f
=3.0hz),129.6,128.2(d,3j
c-f
=
8.1hz),115.8(d,2j
c-f
=22.2hz),109.4,103.6,101.7,79.9,52.3,33.0,24.2;esi-lrms m/z:354.9[m-h]-.
[0092]
实施例14:(e)-4-(苄氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7c-5)的制备
[0093][0094]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,得到化合物7c-5,白色粉末551mg,收率为70%;1h nmr(300mhz,cdcl3)δ11.81(s,1h),7.62(d,j=16.0hz,1h),7.52
–
7.39(m,7h),7.15
–
7.04(m,2h),6.78(d,j=16.0hz,1h),6.73(d,j=2.5hz,1h),6.55(d,j=2.5hz,1h),5.07(s,2h),3.94(s,3h).
13
c nmr(75mhz,cdcl3)δ171.5,165.1,163.2,162.4(d,1j
c-f
=246.0hz),142.6,136.0,133.6(d,4j
c-f
=3.0hz),129.6,129.5(d,5j
c-f
=3.0hz),128.6,128.1(d,3j
c-f
=9.0hz),127.6,115.6(d,2j
c-f
=21.8hz),108.4,104.0,101.1,70.0,52.2;esi-lrms m/z:376.8[m-h]-.
[0095]
实施例15:(e)-4-环丁氧基-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7y-1)的制备
[0096][0097]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为环丁醇,得到化合物7y-1为白色固体299mg,收率为42%;1h nmr(300mhz,cdcl3)δ11.67(s,1h),7.59(d,j=15.9hz,1h),7.49
–
7.38(m,2h),7.05(t,j=8.7hz,2h),6.74(d,j=15.9hz,1h),6.55(d,j=2.5hz,1h),6.30(d,j=2.5hz,1h),4.68(p,j=6.0hz,1h),3.93(s,3h),2.56
–
2.37(m,2h),2.26
–
2.08(m,2h),1.94
–
1.59(m,2h).
13
c nmr(75mhz,cdcl3)δ171.7,165.2,162.6(d,1j
c-f
=240hz),162.3,142.7,133.7(d,4j
c-f
=3hz),129.7(d,5j
c-f
=2.3hz),129.6,128.3(d,3j
c-f
=7.5hz),115.8(d,2j
c-f
=22.5hz),108.7,103.8,101.3,71.9,52.3,30.6,13.4;esi-lrms m/z:340.9[m-h]-.
[0098]
实施例16:(e)-4-(环己氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7y-2)的制备
[0099][0100]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为环己醇,得到化合物7y-2为无色油状液体378mg,收率为49%;1h nmr(300mhz,cdcl3)δ11.65(s,1h),7.60(d,j=15.9hz,1h),7.49
–
7.39(m,2h),7.06(t,j=8.7hz,2h),6.76(d,j=15.9hz,1h),6.60(d,j=2.5hz,1h),6.42(d,j=2.5hz,1h),4.32(m,1h),3.94(s,3h),2.01
(m,2h),1.88
–
1.73(m,2h),1.54
–
1.20(m,6h).
13
c nmr(75mhz,cdcl3)δ171.7,165.3,162.7,162.6(d,1j
c-f
=247.5hz),142.8,133.8(d,4j
c-f
=7.5hz),129.8(d,5j
c-f
=2.3hz),129.7,128.3(d,3j
c-f
=8.3hz),115.7(d,2j
c-f
=22.5hz),109.6,103.6,101.5,75.7,52.4,31.8,25.6,23.8;esi-lrms m/z:368.9[m-h]-.
[0101]
实施例17:(e)-4-(环庚氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7y-3)的制备
[0102][0103]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为环庚醇,得到化合物7y-3为白色固体496mg,收率为62%;1h nmr(300mhz,cdcl3)δ11.68(s,1h),7.60(d,j=15.9hz,1h),7.49
–
7.40(m,2h),7.12
–
7.00(m,2h),6.76(d,j=15.9hz,1h),6.58(d,j=2.1hz,1h),6.38(d,j=2.1hz,1h),4.60
–
4.33(m,1h),3.93(s,3h),2.15
–
1.36(m,12h).
13
c nmr(75mhz,cdcl3)δ171.7,165.3,162.7,162.5(d,1j
c-f
=245.3hz),142.7,133.8(d,4j
c-f
=3.8hz),129.8(d,5j
c-f
=2.3hz),129.6,128.2(d,3j
c-f
=7.5hz),115.8(d,2j
c-f
=21.8hz),109.6,103.4,101.5,100.1,78.2,52.3,33.7,28.4,23.0;esi-lrms m/z:382.9[m-h]-.
[0104]
实施例18:(e)-4-(仲丁氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7y-4)的制备
[0105][0106]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为仲丁醇,得到化合物7y-4为白色固体451mg,收率为63%;1h nmr(300mhz,cdcl3)δ11.66(s,1h),7.61(d,j=15.9hz,1h),7.50
–
7.39(m,2h),7.06(t,j=8.7hz,2h),6.77(d,j=15.9hz,1h),6.60(d,j=2.5hz,1h),6.41(d,j=2.5hz,1h),4.37(h,j=6.1hz,1h),3.94(s,3h),1.85
–
1.53(m,2h),1.83
–
1.59(m,2h),1.33(d,j=6.1hz,3h),0.98(t,j=7.4hz,3h).
13
c nmr(75mhz,cdcl3)δ171.7,165.3,163.1,162.6(d,1j
c-f
=247.5hz),142.8,133.8(d,4j
c-f
=3.0hz),129.8(d,5j
c-f
=2.3hz),129.6,128.3(d,3j
c-f
=8.3hz),115.8(d,2j
c-f
=21.0hz),109.4,103.6,101.5,75.4,52.4,29.2,19.3,9.8;esi-lrms m/z:342.9[m-h]-.
[0107]
实施例19:(e)-2-(4-氟苯乙烯基)-6-羟基-4-(戊-3-基氧基)苯甲酸甲酯(7y-5)的制备
[0108][0109]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为3-戊醇,得到化合物7y-5为无色油状液体395mg,收率为53%;1h nmr(300mhz,cdcl3)δ11.65(s,1h),7.60(d,j=15.9hz,1h),7.49
–
7.39(m,2h),7.06(t,j=8.7hz,2h),6.77(d,j=15.9hz,1h),6.61(d,j=2.5hz,1h),6.41(d,j=2.5hz,1h),4.20(p,j=6.0hz,1h),3.94(s,3h),1.71(qd,j=7.5,5.8hz,4h),0.96(t,j=6.0hz,6h).
13
c nmr(75mhz,cdcl3)δ171.7,165.3,161.6(d,1j
c-f
=247.5hz),163.7,142.8,133.8(d,4j
c-f
=3.8hz),129.9(d,5j
c-f
=3.8hz),129.6,128.3(d,3j
c-f
=8.3hz),115.9(d,2j
c-f
=22.5hz),109.5,103.6,101.5,80.4,52.4,26.2,9.7;esi-lrms m/z:359.2[m h]
.
[0110]
实施例20:(e)-2-(4-氟苯乙烯基)-4-(己-3-基氧基)-6-羟基苯甲酸甲酯(7y-6)的制备
[0111][0112]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为3-己醇,得到化合物7y-6为无色油状液体480mg,收率为62%;1h nmr(300mhz,cdcl3)δ11.66(s,1h),7.61(d,j=15.9hz,1h),7.49
–
7.39(m,2h),7.06(t,j=8.6hz,2h),6.77(d,j=15.9hz,1h),6.61(d,j=2.5hz,1h),6.41(d,j=2.5hz,1h),4.27(m,1h),3.94(s,3h),1.77
–
1.59(m,4h),1.52
–
1.29(m,2h),0.95(q,j=7.1hz,6h).
13
c nmr(75mhz,cdcl3)δ171.7,165.3,163.6,162,6(d,1j
c-f
=247.5hz),142.8,133.8(d,4j
c-f
=3.8hz),129.8(d,5j
c-f
=2.3hz),129.6,128.3(d,3j
c-f
=8.3hz),115.9(d,2j
c-f
=21.0hz),109.5,103.6,101.4,79.1,52.4,35.7,26.7,18.7,14.3,9.7;esi-lrms m/z:370.9[m-h]-.
[0113]
实施例21:(e)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-((四氢-2h-吡喃-4-基)氧基)苯甲酸甲酯(7y-7)的制备
[0114][0115]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料仅将苯甲醇替换为四氢吡喃-4-醇得到化合物7y-7白色晶体411mg,收率为53%;1h nmr(400mhz,cdcl3)δ11.66(s,1h),7.60(d,j=15.9hz,1h),7.50
–
7.38(m,2h),7.06(t,j=8.7hz,2h),6.76(d,j=15.9hz,1h),6.62(d,j=2.5hz,1h),6.43(d,j=2.5hz,1h),4.56(tt,j=7.8,3.8hz,1h),4.03
–
3.95(m,2h),3.94(s,3h),3.60(ddd,j=11.6,8.3,3.2hz,2h),2.05(dd,j=8.6,
4.8hz,2h),1.88
–
1.73(m,2h).
13
c nmr(101mhz,cdcl3)δ171.6,165.2,162.6(d,1j
c-f
=248.5hz),162.0,143.0,133.7(d,4j
c-f
=3.0hz),129.8,129.6(d,5j
c-f
=2.0hz),128.3(d,3j
c-f
=8.1hz),115.8(d,2j
c-f
=21.2hz),109.4,104.1,101.5,71.8,65.1,52.4,31.7;esi-lrms m/z:370.8[m-h]-.
[0116]
实施例22:(e)-4-(2-氟乙氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7y-8)的制备
[0117][0118]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为2-氟乙醇,得到化合物7y-8为白色粉末452mg,收率为65%;1h nmr(300mhz,cdcl3)δ11.64(s,1h),7.60(d,j=15.9hz,1h),7.44(dd,j=10.3,3.7hz,2h),7.06(t,j=8.7hz,2h),6.77(d,j=15.9hz,1h),6.66(d,j=2.6hz,1h),6.43(d,j=2.6hz,1h),4.88
–
4.64(m,2h),4.33
–
4.17(m,2h),3.95(s,3h).
13
c nmr(75mhz,cdcl3)δ171.6,165.2,163.0,162.7(d,1j
c-f
=247.5hz),143.0,133.3(d,4j
c-f
=30.0hz),130.0,129.6(d,5j
c-f
=22.5hz),128.3(d,3j
c-f
=30.0hz),115.8(d,2j
c-f
=21.0hz),108.5,104.6,100.9,81.6(d,1j
c-f
=170.3hz),67.3(d,2j
c-f
=20.3hz),52.4;esi-lrms m/z:332.9[m-h]-.
[0119]
实施例23:(e)-4-(2,2-二氟乙氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7y-9)的制备
[0120][0121]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为2,2-二氟乙醇,得到化合物7y-9为白色固体440mg,收率为60%;1h nmr(400mhz,cdcl3)δ11.65(s,1h),7.60(d,j=16.0hz,1h),7.46(dd,j=8.7,5.4hz,2h),7.07(t,j=8.7hz,2h),6.77(d,j=16.0hz,1h),6.65(d,j=2.6hz,1h),6.43(d,j=2.6hz,1h),6.11(tt,j=54.9,4.0hz,1h),4.23(td,j=12.9,4.0hz,2h),3.96(s,3h).
13
c nmr(101mhz,cdcl3)δ171.5,165.2,162.7(d,1j
c-f
=246.8hz),162.1,143.2,133.6(d,1j
c-f
=4.0hz),130.3,129.3(d,1j
c-f
=2.0hz),128.4(d,3j
c-f
=8.1hz),115.9(d,1j
c-f
=21.2hz),113.4,111.0(t,1j
c-f
=240.0hz),108.1,105.2,101.0,67.1(t,2j
c-f
=30.3hz),52.6;esi-lrms m/z:350.8[m-h]-.
[0122]
实施例24:(e)-2-(4-氟苯乙烯基)-6-羟基-4-(2,2,2-三氟乙氧基)苯甲酸甲酯(7y-10)的制备
[0123][0124]
将化合物6y(600mg,2.08mmol)、无水碳酸钾(431mg,3.12mmol)置于干燥的100ml双颈烧瓶中,氮气保护下,加无水dmf是固体搅拌均匀后,滴加2-碘-1,1,1-三氟乙烷(0.4ml,4.16mmol),升温至80℃。tlc检测反应进程,反应结束后,反应容器放冷至室温,通过硅藻土减压过滤,收集滤液,滤液中加水淬灭反应,以乙酸乙酯萃取,饱和氯化钠溶液洗涤,并用无水硫酸钠干燥,过滤的滤液,依次经过浓缩、柱层析(石油醚:乙酸乙酯=40:1)得到化合物7y-10为白色固体444mg,收率为58%;1h nmr(400mhz,cdcl3)δ11.64(s,1h),7.59(d,j=16.0hz,1h),7.50
–
7.39(m,2h),7.12
–
7.01(m,2h),6.78(d,j=16.0hz,1h),6.67(d,j=2.6hz,1h),6.41(d,j=2.6hz,1h),4.38(q,j=8.0hz,2h),3.96(s,3h).
13
c nmr(101mhz,cdcl3)δ171.5,165.1,162.7(d,1j
c-f
=249.5hz),161.5,143.4,133.5(d,4j
c-f
=3.0hz),130.4,129.1(d,5j
c-f
=2.0hz),128.4(d,3j
c-f
=8.1hz),123.1(q,1j
c-f
=278.8hz),116.0(d,2j
c-f
=21.2hz),108.1,105.6,101.1,65.7(q,2j
c-f
=36.4hz),52.6;esi-lrms m/z:368.8[m-h]-.
[0125]
实施例25:(e)-4-(3-氟丙氧基)-2-(4-氟苯乙烯基)-6-羟基苯甲酸甲酯(7y-11)的制备
[0126][0127]
参照化合物2的合成,以化合物6y(600mg,2.08mmol)为原料,仅将苯甲醇替换为3-氟丙醇,得到化合物7y-11,白色固体406mg,收率为56%;1h nmr(400mhz,cdcl3)δ11.63(s,1h),7.60(d,j=15.9hz,1h),7.50
–
7.42(m,2h),7.10
–
7.01(m,2h),6.76(d,j=15.9hz,1h),6.62(d,j=2.4hz,1h),6.44(d,j=2.4hz,1h),4.65(dt,j=47.0,6.0hz,2h),4.15(t,j=6.0hz,2h),3.94(s,3h),2.19(dp,j=26.0,6.0hz,2h).
13
c nmr(101mhz,cdcl3)δ171.7,165.3,163.4,162.6(d,1j
c-f
=248.5hz),142.9,133.8(d,4j
c-f
=3.0hz),129.8,129.7(d,5j
c-f
=3.0hz),128.3(d,3j
c-f
=8.1hz),115.8(d,1j
c-f
=22.2hz),108.3,104.3,101.0,80.6(d,1j
c-f
=165.6hz),63.9(d,3j
c-f
=5.1hz),52.4,30.4(d,1j
c-f
=20.2hz);esi-lrms m/z:346.8[m-h]-.
[0128]
实施例26:(e)-2-(4-氟苯乙烯基)-6-羟基-4-(3,3,3-三氟丙氧基)苯甲酸甲酯(7y-12)的制备
[0129]
h]-.
[0137]
实施例29:(e)-4-乙氧基-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-1)的制备
[0138][0139]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为乙醇,得到化合物7t-1为白色固体455mg,收率为69%;1h nmr(400mhz,cdcl3)δ11.65(s,1h),7.76(d,j=15.9hz,1h),7.59(dd,j=22.2,8.0hz,4h),6.79(d,j=15.9hz,1h),6.61(s,1h),6.44(s,1h),4.07(q,j=6.9hz,2h),3.94(s,3h),1.44(t,j=6.9hz,3h).
13
c nmr(101mhz,cdcl3)δ171.5,165.4,163.7,142.2,141.1,132.6,129.6(q,2j
c-f
=32.3hz),129.3,126.8,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=272.7hz),108.8,103.9,101.1,64.0 52.4,14.7;esi-lrms m/z:364.8[m-h]-.
[0140]
实施例30:(e)-2-羟基-4-异丙氧基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-2)的制备
[0141][0142]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为异丙醇得到化合物7t-2为白色固体493mg,收率为73%;1h nmr(400mhz,cdcl3)δ11.67(s,1h),7.79(d,j=16.0hz,1h),7.61(dd,j=21.0,8.3hz,4h),6.83(d,j=16.0hz,1h),6.62(d,j=2.4hz,1h),6.46(d,j=2.4hz,1h),4.83
–
4.46(hept,j=6.1hz,1h),3.96(s,3h),1.40(d,j=6.1hz,6h).
13
c nmr(101mhz,cdcl3)δ171.5,165.4,162.8,142.3,141.1,132.6,129.6(q,2j
c-f
=32.3hz),129.3,126.8,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=272.7hz),109.7,103.7,101.9,70.4,52.4,22.0;esi-lrms m/z:378.8[m-h]-.
[0143]
实施例31:(e)-4-(环丙基甲氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-3)的制备
[0144][0145]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为环丙甲醇,得到化合物7t-3为白色固体424mg,收率为60%;1h nmr(400mhz,cdcl3)δ11.64(s,1h),7.76(d,j=16.0hz,1h),7.58(dd,j=22.6,8.3hz,4h),6.80(d,j=16.0hz,1h),6.64(d,j=2.5hz,1h),6.42(d,j=2.5hz,1h),3.94(s,3h),3.84(d,j=7.0hz,2h),1.28(m,1h),0.74
–
0.57(q,j=4.8hz,2h),0.37(q,j=4.8hz,2h).
13
c nmr(101mhz,cdcl3)δ171.5,
165.4,163.7,142.2,141.1,132.6,129.6(q,2j
c-f
=32.3hz),129.3,126.8,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=273.7hz),108.9,103.9,101.1,73.1,52.4,10.1,3.4;esi-lrms m/z:390.8[m-h]-.
[0146]
实施例32:(e)-4-环丁氧基-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-4)的制备
[0147][0148]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料仅将苯甲醇替换为环丁醇,得到化合物7t-14为白色固体346mg,收率为49%;1h nmr(400mhz,cdcl3)δ11.65(s,1h),7.76(d,j=16.0hz,1h),7.59(dd,j=22.0,8.3hz,4h),6.79(d,j=16.0hz,1h),6.57(d,j=2.5hz,1h),6.33(d,j=2.5hz,1h),4.69(p,j=7.1hz,1h),3.93(s,3h),2.55
–
2.41(m,2h),2.26
–
2.11(m,2h),1.96
–
1.67(m,2h).
13
c nmr(101mhz,cdcl3)δ171.5,165.3,162.4,142.2,141.0,132.6,129.6(q,2j
c-f
=32.3hz),129.3,126.8,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=272.7hz),109.1,103.9,101.7,72.0,52.4,30.6,13.4;esi-lrms m/z:390.8[m-h]-.
[0149]
实施例33:(e)-4-(环戊氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-5)的制备
[0150][0151]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为环戊醇,得到化合物7t-5为黄色固体541mg,收率为74%;1h nmr(300mhz,cdcl3)δ11.66(s,1h),7.77(d,j=16.0hz,1h),7.59(q,j=8.5hz,4h),6.80(d,j=16.0hz,1h),6.59(d,j=2.5hz,1h),6.43(d,j=2.5hz,1h),4.81(dt,j=8.5,2.9hz,1h),3.94(s,3h),2.02
–
1.58(m,8h).
13
c nmr(75mhz,cdcl3)δ171.6,165.3,163.1,142.1,141.1,132.7,129.6(q,2j
c-f
=32.3hz),129.3,126.8,125.8(q,3j
c-f
=3.8hz),124.1(q,1j
c-f
=270.2hz),109.8,103.6,102.0,80.0,52.4,33.2,24.2;esi-lrms m/z:404.8[m-h]-.
[0152]
实施例34:(e)-4-(环己氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-6)的制备
[0153][0154]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为环己醇得到化合物7t-16为无色油状液体409mg,收率为54%;1h nmr(400mhz,cdcl3)δ11.64(s,1h),7.77(d,j=16.0hz,1h),7.59(dd,j=19.9,8.4hz,4h),6.81(d,j=16.0hz,1h),
6.61(d,j=2.4hz,1h),6.45(d,j=2.4hz,1h),4.45
–
4.17(m,1h),3.94(s,3h),1.91(m,4h),1.69
–
1.25(m,6h).
13
c nmr(101mhz,cdcl3)δ171.6,165.4,162.8,142.2,141.1,132.7,129.6(q,2j
c-f
=33.3hz),129.3,126.8,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=280.8hz),109.9,103.6,101.8,75.8,52.4,31.7,25.6,23.8;esi-lrms m/z:418.8[m-h]-.
[0155]
实施例35:(e)-4-(环庚氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-7)的制备
[0156][0157]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为环庚醇,得到化合物7t-17为无色油状液体579mg,收率为74%;1h nmr(300mhz,cdcl3)δ11.66(s,1h),7.77(d,j=15.9hz,1h),7.59(q,j=8.5hz,4h),6.80(d,j=15.9hz,1h),6.59(d,j=2.5hz,1h),6.40(d,j=2.5hz,1h),4.48(t,j=12.2hz,1h),3.94(s,3h),2.18
–
1.35(m,12h).
13
c nmr(75mhz,cdcl3)δ171.6,165.4,162.8,142.2,141.1,132.6,130.0(q,2j
c-f
=32.3hz),129.3,126.8,125.8(q,3j
c-f
=3.8hz),123.9(q,1j
c-f
=270.2hz),110.0,103.5,101.9,78.3,52.4,33.7,28.5,23.1;esi-lrms m/z:432.9[m-h]-.
[0158]
实施例36:(e)-4-(苄氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-8)的制备
[0159][0160]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料得到化合物7t-8,白色粉末578mg,收率为75%;1h nmr(400mhz,cdcl3)δ11.67(s,1h),7.78(d,j=15.9hz,1h),7.60(dd,j=23.5,8.2hz,4h),7.49
–
7.32(m,5h),6.80(d,j=15.9hz,1h),6.72(d,j=2.2hz,1h),6.55(d,j=2.2hz,1h),5.11(s,2h),3.95(s,3h).
13
c nmr(101mhz,cdcl3)δ171.5,165.3,163.4,142.3,141.0,136.1,132.5,129.6(q,2j
c-f
=32.3hz),129.5,128.8,128.4,127.7,126.9,125.8(q,3j
c-f
=4.0hz),124.7(q,1j
c-f
=273.7hz),109.0,104.3,101.6,70.3,52.4;
[0161]
esi-lrms m/z:426.8[m-h]-.
[0162]
实施例37:(e)-4-(仲丁氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-9)的制备
[0163][0164]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为仲
丁醇,得到化合物7t-9为白色固体419mg,收率为59%;1h nmr(400mhz,cdcl3)δ11.66(s,1h),7.80(d,j=16.0hz,1h),7.62(dd,j=19.5,8.4hz,4h),6.83(d,j=16.0hz,1h),6.63(d,j=2.4hz,1h),6.46(d,j=2.4hz,1h),4.40(h,j=6.0hz,1h),3.97(s,3h),1.74(m,2h),1.36(d,j=6.0hz,3h),1.01(t,j=7.5hz,3h).
13
c nmr(101mhz,cdcl3)δ171.6,165.4,163.2,142.3,141.1,132.7,129.7(q,2j
c-f
=32.3hz),129.3,126.9,125.9(q,3j
c-f
=3.0hz),124.4(q,1j
c-f
=273.7hz),109.8,103.7,101.8,75.5,52.4,29.2,19.3,9.8;esi-lrms m/z:392.9[m-h]-.
[0165]
实施例38:(e)-2-羟基-4-(戊-3-基氧基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-10)的制备
[0166][0167]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为3-戊醇,得到化合物7t-10为白色晶体434mg,收率为59%;1h nmr(400mhz,cdcl3)δ11.68(s,1h),7.80(d,j=16.0hz,1h),7.62(q,j=8.4hz,4h),6.84(d,j=16.0hz,1h),6.65(d,j=2.5hz,1h),6.46(d,j=2.5hz,1h),4.23(p,j=5.9hz,1h),3.96(s,3h),1.83
–
1.63(m,4h),0.99(t,j=7.4hz,6h).
13
c nmr(101mhz,cdcl3)δ171.5,165.4,163.7,142.3,141.1,132.7,129.6(q,2j
c-f
=32.3hz),129.2,126.8,125.9(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),109.8,103.6,101.8,80.5,52.4,26.2,9.6;esi-lrms m/z:406.8[m-h]-.
[0168]
实施例39:(e)-4-(己-3-基氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-11)的制备
[0169][0170]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为3-己醇,得到化合物7t-11为无色油状液体486mg,收率为64%;1h nmr(300mhz,cdcl3)δ11.66(s,1h),7.78(d,j=16.0hz,1h),7.60(q,j=8.6hz,4h),6.81(d,j=16.0hz,1h),6.63(d,j=2.5hz,1h),6.44(d,j=2.5hz,1h),4.34
–
4.19(m,1h),3.94(s,3h),1.73
–
1.32(m,6h),0.95(q,j=7.2hz,6h).
13
c nmr(75mhz,cdcl3)δ171.6,165.4,163.7,142.2,141.1,132.6,129.6(q,2j
c-f
=32.3hz),129.3,126.8,125.8(q,3j
c-f
=3.8hz),124.3(q,1j
c-f
=270.2hz),109.8,103.6,101.7,79.2,52.4,35.6,26.7,18.7,14.2,9.6;esi-lrms m/z:420.8[m-h]-.
[0171]
实施例40:(e)-4-(2-氟乙氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-12)的制备
[0172][0173]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为2-氟乙醇,得到化合物7t-12为白色粉末469mg,收率为68%;1h nmr(400mhz,cdcl3)δ11.64(s,1h),7.76(d,j=16.0hz,1h),7.59(dd,j=22.2,8.3hz,4h),6.81(d,j=16.0hz,1h),6.67(d,j=2.3hz,1h),6.45(d,j=2.3hz,1h),4.88
–
4.66(m,2h),4.32
–
4.19(m,2h),3.95(s,3h).
13
c nmr(101mhz,cdcl3)δ171.5,165.3,163.0,142.4,141.0,132.3,129.7(q,2j
c-f
=32.3hz),129.6,126.9,125.9(q,3j
c-f
=3.0hz),124.4(q,1j
c-f
=272.2hz),108.8,104.6,101.2,81.5(d,1j
c-f
=172.7hz),67.3(d,2j
c-f
=21.2hz),52.5;esi-lrms m/z:382.8[m-h]-.
[0174]
实施例41:(e)-4-(2,2-二氟乙氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-13)的制备
[0175][0176]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为2,2-二氟乙醇,得到化合物7t-13为白色粉末456mg,收率为63%;1h nmr(400mhz,cdcl3)δ11.64(s,1h),7.76(d,j=16.0hz,1h),7.60(dd,j=22.0,8.4hz,4h),6.81(d,j=16.0hz,1h),6.66(d,j=2.6hz,1h),6.45(d,j=2.6hz,1h),6.10(tt,j=54.9,4.0hz,1h),4.23(td,j=12.9,4.0hz,2h),3.96(s,3h).
13
c nmr(101mhz,cdcl3)δ171.4,165.3,162.1,142.7,140.8,132.1,129.9(q,2j
c-f
=32.3hz),129.9,126.9,125.7(q,3j
c-f
=3.0hz),124.4(q,1j
c-f
=272.7hz),126.9,113.4(t,1j
c-f
=242.4hz),108.4,105.2,101.4,67.2(t,2j
c-f
=30.3hz),52.6;esi-lrms m/z:400.8[m-h]-.
[0177]
实施例42:(e)-2-羟基-4-(2,2,2-三氟乙氧基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-14)的制备
[0178][0179]
参照化合物7y-10的合成,以化合物6t(700mg,1.80mmol)为原料,得到化合物7t-14,白色晶体507mg,收率为67%;1h nmr(400mhz,cdcl3)δ11.67(s,1h),7.78(d,j=16.0hz,1h),7.62(dd,j=21.8,8.3hz,4h),6.85(d,j=16.0hz,1h),6.72(d,j=2.5hz,1h),6.47(d,j=2.5hz,1h),4.42(q,j=7.9hz,2h),3.99(s,3h).
13
c nmr(101mhz,cdcl3)δ171.3,165.2,161.6,142.8,140.8,131.9,130.1,129.9(q,2j
c-f
=32.3hz),126.9,125.9(q,3j
c-f
=
4.0hz),124.3(q,1j
c-f
=272.7hz),123.1(q,1j
c-f
=278.8hz),108.5,105.7,101.5,65.5(q,2j
c-f
=36.4hz),52.7;esi-lrms m/z:418.8[m-h]-.
[0180]
实施例43:(e)-4-(3-氟丙氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-15)的制备
[0181][0182]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为3-氟丙醇,得到化合物7t-15为白色粉末509mg,收率为71%;1h nmr(400mhz,cdcl3)δ11.65(s,1h),7.76(d,j=16.0hz,1h),7.59(dd,j=20.7,8.4hz,4h),6.80(d,j=16.0hz,1h),6.63(d,j=2.5hz,1h),6.46(d,j=2.5hz,1h),4.65(dt,j=47.0,6.0hz,2h),4.15(t,j=6.0hz,2h),3.94(s,3h),2.19(dp,j=26.0,6.0hz,2h).
13
c nmr(101mhz,cdcl3)δ171.5,165.3,163.5,142.3,141.0,132.5,129.6(q,2j
c-f
=32.3hz),129.4,126.8,125.9(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),108.5,104.2,101.3,80.6(d,1j
c-f
=165.6hz),64.0(d,3j
c-f
=5.1hz),52.4,30.3(d,2j
c-f
=20.2hz);esi-lrms m/z:396.8[m-h]-.
[0183]
实施例44:(e)-2-羟基-6-(4-(三氟甲基)苯乙烯基)-4-(3,3,3-三氟丙氧基)苯甲酸甲酯(7t-16)的制备
[0184][0185]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为3,3,3-三氟丙醇,得到化合物7t-16为白色固体446mg,收率为57%;1h nmr(400mhz,cdcl3)δ11.65(s,1h),7.76(d,j=16.0hz,1h),7.60(dd,j=20.5,8.4hz,4h),6.81(d,j=16.0hz,1h),6.62(d,j=2.6hz,1h),6.44(d,j=2.6hz,1h),4.24(t,j=6.5hz,2h),3.95(s,3h),2.65(qt,j=10.5,6.5hz,2h).
13
c nmr(101mhz,cdcl3)δ171.4,165.3,162.7,142.5,140.9,132.3,129.8(q,2j
c-f
=32.3hz),129.6,126.9,125.9(q,1j
c-f
=277.8hz),124.4(q,1j
c-f
=272.7hz),125.9(q,3j
c-f
=3.0hz),108.5,104.7,101.2,61.2(q,3j
c-f
=4.0hz),52.5,34.1(q,2j
c-f
=29.3hz);esi-lrms m/z:432.8[m-h]-.
[0186]
实施例45:(e)-4-(4-氟丁氧基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-17)的制备
[0187]
[0188]
参照化合物7y-10的合成,以化合物6t(700mg,1.80mmol)为原料得到化合物7t-17,白色固体534mg,收率为72%;1h nmr(400mhz,cdcl3)δ11.65(s,1h),7.76(d,j=15.9hz,1h),7.59(dd,j=20.0,8.1hz,4h),6.80(d,j=15.9hz,1h),6.62(d,j=2.1hz,1h),6.44(d,j=2.1hz,1h),4.50(dd,j=29.1,23.4hz,2h),4.05(t,j=5.5hz,2h),3.94(s,3h),2.05
–
1.76(m,4h).
13
c nmr(101mhz,cdcl3)δ171.5,165.3,163.7,142.2,141.0,132.5,129.6(q,2j
c-f
=32.3hz),129.4,126.8,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=273.7hz),108.7,104.0,101.1,83.8(d,1j
c-f
=32.3hz),67.7,52.4,27.2(d,2j
c-f
=20.2hz),25.3(d,3j
c-f
=4.0hz);esi-lrms m/z:410.8[m-h]-.
[0189]
实施例46:(e)-2-羟基-4-(4,4,4-三氟丁氧基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(7t-18)的制备
[0190][0191]
参照化合物2的合成,以化合物6t(700mg,1.80mmol)为原料,仅将苯甲醇替换为4,4,4-三氟丁醇,得到化合物7t-18为白色固体533mg,收率为66%;1h nmr(400mhz,cdcl3)δ11.67(s,1h),7.76(d,j=16.0hz,1h),7.60(dd,j=20.5,8.4hz,4h),6.81(d,j=16.0hz,1h),6.62(d,j=2.5hz,1h),6.43(d,j=2.5hz,1h),4.06(t,j=6.0hz,2h),3.95(s,3h),2.45
–
2.24(m,2h),2.08(m,2h).
13
c nmr(101mhz,cdcl3)δ171.5,165.3,163.3,142.4,141.0,132.4,129.7(q,2j
c-f
=32.3hz),129.2,126.9,125.9(q,3j
c-f
=4.0hz),125.7(q,1j
c-f
=278.8hz),124.4(q,1j
c-f
=273.7hz),108.6,104.4,101.1,66.4,52.5,30.8(q,2j
c-f
=29.3hz),22.1(q,3j
c-f
=3.0hz);esi-lrms m/z:446.7[m-h]-.
[0192]
实施例47:(e)-4-乙氧基-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8c-1)的制备
[0193][0194]
向含磁力搅拌子的50ml支口烧瓶中加入化合物7c-1(484mg,1.53mmol),以20ml的无水甲苯溶解,冰水浴条件下将氢化钠(79.6mg,1.99mmol,60%)加入到反应液中,并以氮气保护,20min后,待反应液气泡消失,将1-溴-3-甲基-2-丁烯(0.2ml,1.99mmol)缓慢滴入,同时将温度升高至65℃。tlc检测反应进程结束后,将反应容器放冷至室温,以饱和氯化铵溶液淬灭反应,加入乙酸乙酯萃取,有机层以无水硫酸钠干燥,过滤,收集滤液并浓缩,残渣以硅胶柱层析纯化(石油醚:乙酸乙酯=150:1),得到化合物8c-1为白色固体170mg,收率为34%;1h nmr(300mhz,cdcl3)δ11.68(s,1h),7.62(d,j=16.0hz,1h),7.52
–
7.40(m,2h),7.16
–
6.99(m,2h),6.71(d,j=15.9hz,1h),6.56(s,1h),5.36
–
5.18(m,1h),4.15(q,j=7.0hz,2h),3.93(s,3h),3.40(d,j=7.2hz,2h),1.81(s,3h),1.69(s,3h),1.46(t,j=7.0hz,3h).
13
c nmr(75mhz,cdcl3)δ172.0,162.5(d,1j
c-f
=247.5hz),161.7,160.9,140.1,
133.9(d,4j
c-f
=3.8hz),131.7,130.6(d,5j
c-f
=2.3hz),128.9,128.2(d,3j
c-f
=8.3hz),122.2,116.9,115.8(d,2j
c-f
=23.3hz),110.1,104.4,103.8,64.0,52.4,26.0,22.4,18.0,15.0;esi-lrms m/z:382.9[m-h]-.
[0195]
实施例48:(e)-6-(4-氟苯乙烯基)-2-羟基-4-异丙氧基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8c-2)的制备
[0196][0197]
参照化合物8c-1的合成,以化合物7c-2(555mg,1.68mmol)为原料得到化合物8c-2,黄色固体208mg,收率为31%;1h nmr(400mhz,cdcl3)δ11.70(s,1h),7.63(d,j=15.9hz,1h),7.47(dd,j=8.7,5.4hz,2h),7.06(t,j=8.7hz,2h),6.69(d,j=15.9hz,1h),6.58(s,1h),5.29
–
5.21(m,2h),4.73(hept,j=6.0hz,1h),3.93(s,3h),3.37(d,j=8.0hz,2h),1.81(s,3h),1.69(s,3h),1.40(s,4h),1.38(s,4h).
13
c nmr(101mhz,cdcl3)δ172.0,162.5(d,1j
c-f
=248.5hz),162.0,160.1,140.0,134.0(d,4j
c-f
=3.0hz),131.5,130.8(d,5j
c-f
=2.0hz),128.8,128.2(d,3j
c-f
=8.1hz),122.4,117.9,115.8(d,2j
c-f
=21.2hz),105.0,104.2,70.3,52.3,29.8,26.0,22.5,22.4,18.1;esi-lrms m/z:396.8[m-h]-.
[0198]
实施例49:(e)-4-(环丙基甲氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8c-3)的制备
[0199][0200]
参照化合物8c-1的合成,以化合物7c-3(472mg,1.38mmol)为原料得到化合物8c-3,黄色固体243mg,收率为43%;1h nmr(300mhz,cdcl3)δ11.68(s,1h),7.61(d,j=15.9hz,1h),7.52
–
7.40(m,2h),7.12
–
6.99(m,2h),6.70(d,j=15.9hz,1h),6.52(s,1h),5.35
–
5.20(m,1h),3.93(d,j=7.0hz,2h),3.93(s,3h),3.42(d,j=7.2hz,2h),1.83(s,3h),1.69(s,3h),1.31
–
1.24(m,1h),0.65(q,j=5.0hz,2h),0.37(q,j=5.0hz,2h).
13
c nmr(75mhz,cdcl3)δ171.9,162.3(d,1j
c-f
=245.3hz),161.6,160.9,140.0,133.7(d,4j
c-f
=3.8hz),131.6,130.5(d,5j
c-f
=2.3hz),128.7,128.1(d,3j
c-f
=7.5hz),122.1,116.9,115.7(d,2j
c-f
=21.8hz),104.3,103.8,77.5,77.0,76.6,72.9,52.2,25.9,22.3,17.9,10.3,3.2;esi-lrms m/z:408.9[m-h]-.
[0201]
实施例50:(e)-4-(环戊氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8c-4)的制备
[0202]
[0203]
参照化合物8c-1的合成,以化合物7c-4(473mg,1.33mmol)为原料得到化合物8c-4,黄色固体125mg,收率为22%;1h nmr(300mhz,cdcl3)δ11.72(s,1h),7.64(d,j=15.9hz,1h),7.53
–
7.42(m,2h),7.15
–
7.00(m,2h),6.71(d,j=15.9hz,1h),6.59(s,1h),5.26(m,1h),4.95(p,j=4.1hz,1h),3.93(s,3h),3.38(d,j=7.2hz,2h),1.81(s,3h),1.70(s,3h),2.01
–
1.65(m,8h).
13
c nmr(75mhz,cdcl3)δ172.0,164.1,162.4(d,1j
c-f
=246.0hz),161.8,160.8,160.1,139.9(d,4j
c-f
=3.0hz),131.5,130.8(d,5j
c-f
=3.0hz),128.8,128.1(d,3j
c-f
=8.3hz),122.4,117.4,115.7(d,2j
c-f
=21.8hz),104.9,104.0,79.6,52.3,33.2,25.9,24.1,22.4,17.9;esi-lrms m/z:425.3[m h]
.
[0204]
实施例51:(e)-4-(苄氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8c-5)的制备
[0205][0206]
参照化合物8c-1的合成,以化合物7c-5(524mg,1.38mmol)为原料得到化合物8c-5,黄色固体260mg,收率为42%;1h nmr(400mhz,cdcl3)δ11.73(s,1h),7.63(d,j=15.9hz,1h),7.52
–
7.32(m,7h),7.09(dd,j=12.1,5.3hz,2h),6.67(d,j=15.9hz,2h),6.66(s,1h),5.29(t,j=7.0hz,1h),5.21(s,2h),3.94(s,3h),3.47(d,j=7.0hz,2h),1.74(s,3h),1.71(s,3h).
13
c nmr(101mhz,cdcl3)δ171.9,162.5(d,1j
c-f
=247.5hz),161.8,160.6,140.2,136.8,133.8(d,4j
c-f
=4.0hz),131.9,130.5(d,5j
c-f
=2.0hz),129.1,128.2,128.1(d,3j
c-f
=8.1hz),127.4,122.2,117.3,115.8(d,2j
c-f
=22.2hz),104.8,104.2,70.2,52.3,25.9,22.5,17.9;esi-lrms m/z:447.2[m h]
.
[0207]
实施例52:(e)-4-环丁氧基-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8y-1)的制备
[0208][0209]
参照化合物8c-1的合成,以化合物7y-1(283mg,0.83mmol)为原料得到化合物8y-1,黄色固体139mg,收率为41%;1h nmr(300mhz,cdcl3)δ11.67(s,1h),7.61(d,j=15.9hz,1h),7.51
–
7.41(m,2h),7.07(t,j=8.7hz,2h),6.68(d,j=15.9hz,1h),6.42(s,1h),5.31
–
5.20(m,1h),4.78(p,j=6.0hz,1h),3.93(s,3h),3.44
–
3.30(d,j=6.0hz,2h),2.49(m,2h),2.31
–
2.11(m,2h),1.91
–
1.67(m,8h).
13
c nmr(75mhz,cdcl3)δ172.0,163.5(d,1j
c-f
=247.5hz),161.8,159.7,140.0,133.9(d,4j
c-f
=3.8hz),131.7,130.7(d,5j
c-f
=2.3hz),128.9,128.2(d,3j
c-f
=8.3hz),122.2,117.1,115.8(d,2j
c-f
=22.5hz),104.6,104.4,71.9,52.3,30.9,26.0,22.5,18.0,13.5;esi-lrms m/z:411.2[m h]
.
[0210]
实施例53:(e)-4-(环己氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8y-2)的制备
nmr(75mhz,cdcl3)δ172.0,163.5(d,1j
c-f
=247.5hz),162.0,160.3,140.0,133.9(d,4j
c-f
=3.8hz),131.5,130.8(d,5j
c-f
=3.0hz),128.8,128.2(d,3j
c-f
=7.5hz),122.4,117.7,115.7(d,2j
c-f
=22.5hz),104.7,104.0,75.0,52.3,29.4,26.0,22.5,19.5,18.0,9.8;esi-lrms m/z:410.9[m-h]-.
[0219]
实施例56:(e)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(戊基-3-基氧基)苯甲酸甲酯(8y-5)的制备
[0220][0221]
参照化合物8c-1的合成,以化合物7y-5(377mg,1.05mmol)为原料得到化合物8y-5,黄色油状液体197mg,产率44%;1h nmr(300mhz,cdcl3)δ11.72(s,1h),7.64(d,j=15.9hz,1h),7.53
–
7.41(m,2h),7.10
–
7.04(m,2h),6.70(d,j=15.9hz,1h),6.65(s,1h),5.30
–
5.19(m,1h),4.41-4.33(p,j=6.0hz,1h),3.93(s,3h),3.41(d,j=7.0hz,2h),1.80(s,3h),1.75(qd,j=7.5,6.0hz,4h),1.69(s,3h),0.98(t,j=6.0hz,7h).
13
c nmr(75mhz,cdcl3)δ172.0,162.5(d,1j
c-f
=247.5hz),162.0,160.6,140.0,133.9(d,4j
c-f
=3.0hz),131.4,130.9(d,5j
c-f
=2.3hz),128.7,128.2(d,3j
c-f
=8.3hz),122.4,117.5,115.8(d,2j
c-f
=23.3hz),104.6,103.9,79.7,52.3,26.2,25.9,22.5,18.0,9.5;esi-lrms m/z:424.9[m-h]-.
[0222]
实施例57:(e)-6-(4-氟苯乙烯基)-4-(己-3-基氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8y-6)的制备
[0223][0224]
参照化合物8c-1的合成,以化合物7y-6(456mg,1.22mmol)为原料得到化合物8y-6,黄色油状液体210mg,收率为39%;1h nmr(300mhz,cdcl3)δ11.68(s,1h),7.62(d,j=15.9hz,1h),7.46(m,2h),7.06(t,j=8.6hz,3h),6.68(d,j=15.9hz,1h),6.54(s,1h),5.22(m,1h),4.48
–
4.33(m,1h),3.92(s,3h),3.38(d,j=7.1hz,2h),1.79(s,3h),1.75
–
1.70(m,4h),1.67(s,3h),1.43(m,2h),1.02
–
0.88(q,j=7.1hz,6h).
13
c nmr(75mhz,cdcl3)δ172.0,162.5(d,1j
c-f
=247.5hz),162.0,160.7,140.0,134.0(d,4j
c-f
=3.0hz),131.5,130.9(d,5j
c-f
=2.3hz),128.8,128.2(d,3j
c-f
=7.5hz),122.4,117.5,115.9(d,2j
c-f
=21.8hz),104.6,103.9,78.4,52.3,35.8,26.8,26.0,22.5,18.7,18.0,14.4,9.6;esi-lrms m/z:441.3[m h]
.
[0225]
实施例58:(e)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-((四氢-2h-吡喃-4-基)氧基)苯甲酸甲酯(8y-7)的制备
[0226][0227]
参照化合物8c-1的合成,以化合物7y-7(385mg,1.03mmol)为原料得到化合物8y-7,黄色油状液体132mg,收率为29%;1h nmr(400mhz,cdcl3)δ11.70(s,1h),7.61(d,j=15.9hz,1h),7.51
–
7.38(m,2h),7.06(t,j=8.7hz,2h),6.66(d,j=15.9hz,1h),6.54(s,1h),5.24(t,j=7.6hz,1h),4.77
–
4.61(m,1h),4.02
–
3.95(m,2h),3.93(s,3h),3.64(ddd,j=11.3,7.4,3.5hz,2h),3.41(d,j=7.0hz,2h),2.06(ddd,j=16.2,6.8,3.5hz,2h),1.86(ddd,j=13.9,7.4,3.7hz,2h),1.80(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ171.9,162.5(d,1j
c-f
=248.5hz),162.1,159.2,140.1,133.7(d,4j
c-f
=4.0hz),131.8,130.6(d,5j
c-f
=3.0hz),129.0,128.2(d,3j
c-f
=8.1hz),122.3,118.0,115.8(d,2j
c-f
=21.2hz),104.8,104.5,71.3,64.9,52.4,31.9,25.9,22.5,18.1;esi-lrms m/z:441.2[m h]
.
[0228]
实施例59:(e)-4-(2-氟乙氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8y-8)的制备
[0229][0230]
参照化合物8c-1的合成,以化合物7y-8(436mg,1.30mmol)为原料得到化合物8y-8,黄色固体131mg,收率为25%;1h nmr(400mhz,cdcl3)δ11.69(s,1h),7.61(d,j=15.9hz,1h),7.46(dd,j=8.7,5.4hz,2h),7.06(t,j=8.7hz,2h),6.71(d,j=15.9hz,1h),6.55(s,1h),5.25(t,j=7.2hz,1h),4.91
–
4.64(m,2h),4.42
–
4.21(m,2h),3.94(s,3h),3.42(d,j=7.2hz,2h),1.81(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ171.9,162.9(d,1j
c-f
=247.5hz),161.8,160.3,140.3,133.8(d,4j
c-f
=3.8hz),131.9,130.4(d,5j
c-f
=4.0hz),129.1,128.2(d,3j
c-f
=8.3hz),122.0,117.4,115.8(d,2j
c-f
=18.8hz),105.2,103.8,81.8(d,1j
c-f
=171.0hz),67.6(d,2j
c-f
=21.0hz),52.4,25.9,22.4,17.9;esi-lrms m/z:400.8[m-h]-.
[0231]
实施例60:(e)-4-(2,2-二氟乙氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8y-9)的制备
[0232][0233]
参照化合物8c-1的合成,以化合物7y-9(425mg,1.21mmol)为原料得到化合物8y-9,白色固体188mg,收率为37%;1h nmr(300mhz,cdcl3)δ11.70(s,1h),7.61(d,j=16.0hz,
三氟丙氧基)苯甲酸甲酯(8y-12)的制备
[0241][0242]
参照化合物8c-1的合成,以化合物7y-12(384mg,1.00mmol)为原料得到化合物8y-12,黄色固体149mg,收率为33%;1h nmr(300mhz,cdcl3)δ11.67(s,1h),7.62(d,j=16.0hz,1h),7.50
–
7.43(m,2h),7.07(t,j=8.7hz,2h),7.05(d,j=8.7hz,1h),6.72(d,j=16.0hz,1h),6.54(s,1h),5.21(t,j=4.7hz,1h),4.31(t,j=6.5hz,2h),3.94(s,3h),3.38(d,j=7.1hz,2h),2.69(qd,j=10.5,5.2hz,2h),1.78(s,3h),1.68(s,3h).
13
c nmr(75mhz,cdcl3)δ171.9,162.6(d,1j
c-f
=246.8hz),161.8,159.9,140.4,133.8,133.7(d,4j
c-f
=3.8hz),132.0,130.3(d,5j
c-f
=2.3hz),129.3,128.2(d,3j
c-f
=8.3hz),122.0,117.3,115.9(d,2j
c-f
=21.8hz),105.3,103.3,61.3(q,3j
c-f
=3.8hz),52.5,34.3(q,2j
c-f
=34.3hz),29.9,25.9,22.3,17.9;esi-lrms m/z:450.8[m-h]-.
[0243]
实施例64:(e)-4-(3-氟丙氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸甲酯(8y-13)的制备
[0244][0245]
参照化合物8c-1的合成,以化合物7y-13(506mg,1.40mmol)为原料得到化合物8y-13,白色固体271mg,收率为45%;1h nmr(400mhz,cdcl3)δ11.68(s,1h),7.62(d,j=15.9hz,1h),7.46(dd,j=8.2,5.6hz,2h),7.07(t,j=8.6hz,2h),6.72(d,j=15.9hz,1h),6.57(s,1h),5.23(t,j=6.7hz,1h),4.66
–
4.39(m,2h),4.14(t,j=5.6hz,2h),3.93(s,3h),3.40(d,j=6.9hz,2h),2.05
–
1.85(m,4h),1.80(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ172.0,162.6(d,1j
c-f
=248.5hz),161.7,160.8,140.3,133.9(d,4j
c-f
=3.0hz),131.7,130.6(d,5j
c-f
=2.0hz),129.0,128.2(d,3j
c-f
=8.1hz),122.3,116.9,115.9(d,2j
c-f
=248.5hz),104.6,103.6,83.8(d,1j
c-f
=165.6hz),67.7,52.3,27.3(d,2j
c-f
=19.2hz),25.9,25.5(d,3j
c-f
=5.1hz),22.4,18.0;esi-lrms m/z:428.9[m-h]-.
[0246]
实施例65:(e)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(4,4,4-三氟丁氧基)苯甲酸甲酯(8y-14)的制备
[0247]
[0248]
参照化合物8c-1的合成,以化合物7y-14(496mg,1.25mmol)为原料得到化合物8y-14,白色晶体232mg,收率为41%;1h nmr(400mhz,cdcl3)δ11.68(s,1h),7.62(d,j=15.9hz,1h),7.50
–
7.43(m,2h),7.06(t,j=8.7hz,2h),6.71(d,j=15.9hz,1h),6.54(s,1h),5.23
–
5.08(m,1h),4.15(t,j=6.2hz,2h),3.94(s,3h),3.38(d,j=6.9hz,2h),2.41
–
2.28(m,2h),2.10(dt,j=16.2,5.8hz,2h),1.78(s,3h),1.68(s,3h).
13
c nmr(101mhz,cdcl3)δ172.0,162.6(d,1j
c-f
=248.5hz),161.7,160.4,147.8,140.4,133.8(d,4j
c-f
=3.0hz),131.9,130.4(d,5j
c-f
=2.0hz),129.2,128.2(d,3j
c-f
=7.1hz),124.4(q,1j
c-f
=276.7hz),122.3,117.0,115.9(d,2j
c-f
=22.2hz),104.9,103.5,66.4,52.5,30.8(q,2j
c-f
=29.3hz),25.9,22.4(d,3j
c-f
=3.0hz),22.4,17.9;esi-lrms m/z:464.8[m-h]-.
[0249]
实施例66:(e)-4-乙氧基-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-1)的制备
[0250][0251]
参照化合物8c-1的合成,以化合物7t-1(412mg,1.1mmol)为原料得到化合物8t-1,白色固体176mg,收率为36%;1h nmr(400mhz,cdcl3)δ11.67(s,1h),7.79(d,j=16.0hz,1h),7.60(dd,j=18.1,8.4hz,4h),6.75(d,j=16.0hz,1h),6.58(s,1h),5.26(t,j=7.2hz,1h),4.16(q,j=7.0hz,2h),3.93(s,3h),3.41(d,j=7.2hz,2h),1.81(s,3h),1.69(s,3h),1.47(t,j=7.0hz,3h).
13
c nmr(101mhz,cdcl3)δ171.8,161.8,161.0,141.2,139.6,133.5,131.8,129.5(q,2j
c-f
=32.3hz),128.5,126.8,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.2,117.5,104.6,104.0,64.1,52.4,26.0,22.4,18.0,15.0;esi-lrms m/z:432.8[m-h]-.
[0252]
实施例67:(e)-2-羟基-4-异丙氧基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-2)的制备
[0253][0254]
参照化合物8c-1的合成,以化合物7t-2(488mg,1.28mmol)为原料得到化合物8t-2,黄色油状物247mg,收率为43%;1h nmr(400mhz,cdcl3)δ11.72(s,1h),7.81(d,j=15.9hz,1h),7.61(q,j=8.4hz,4h),6.75(d,j=15.9hz,1h),6.60(s,1h),5.27(t,j=7.1hz,1h),4.84
–
4.67(m,1h),3.93(s,3h),3.40(d,j=7.3hz,2h),1.82(s,3h),1.70(s,3h),1.41(d,j=6.0hz,6h).
13
c nmr(101mhz,cdcl3)δ171.8,162.1,160.1,141.2,139.5,133.6,131.6,129.4(q,2j
c-f
=32.3hz),128.5,126.8,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=272.7hz),122.2,118.3,105.1,104.1,70.3,52.4,25.9,22.5,22.3,18.0;esi-lrms m/z:446.8[m-h]-.
[0255]
实施例68:(e)-4-(环丙基甲氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三
氟甲基)苯乙烯基)苯甲酸甲酯(8t-3)的制备
[0256][0257]
参照化合物8c-1的合成,以化合物7t-3(406mg,1.03mmol)为原料得到化合物8t-3,白色固体172mg,收率为36%;1h nmr(400mhz,cdcl3)δ11.68(s,1h),7.79(d,j=16.0hz,1h),7.60(q,j=8.4hz,4h),6.75(d,j=16.0hz,1h),6.54(s,1h),5.29(t,j=7.2hz,1h),3.94(m,5h),3.44(d,j=7.2hz,2h),1.84(s,3h),1.70(s,3h),1.31(m,1h),0.67(q,j=5.0hz,2h),0.40(q,j=5.0hz,2h).
13
c nmr(101mhz,cdcl3)δ171.8,161.8,161.1,141.2,139.6,133.4,131.8,129.5(q,2j
c-f
=32.3hz),128.5,126.8,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.2,117.6,104.5,104.1,73.1,52.4,26.0,22.5,18.0,10.4,3.3;esi-lrms m/z:458.8[m-h]-.
[0258]
实施例69:(e)-4-环丁氧基-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-4)的制备
[0259][0260]
参照化合物8c-1的合成,以化合物7t-4(327mg,0.8mmol)为原料得到化合物8t-4,黄色油状液体150mg,收率为39%;1h nmr(400mhz,cdcl3)δ11.64(s,1h),7.78(d,j=15.9hz,1h),7.60(q,j=8.4hz,4h),6.71(d,j=15.9hz,1h),6.42(s,1h),5.33
–
5.14(m,1h),4.86
–
4.70(m,1h),3.92(s,3h),3.39(d,j=6.9hz,3h),2.55
–
2.41(m,2h),2.26
–
2.11(m,3h),1.81(s,3h),1.80
–
1.70(m,2h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ171.8,161.9,159.7,141.2,139.5,133.5,131.9,129.5(q,2j
c-f
=32.3hz),128.5,126.8,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=270.7hz),122.1,117.6,104.8,104.5,71.9,52.4,30.9,26.0,22.5,18.1,13.5;esi-lrms m/z:458.9[m-h]-.
[0261]
实施例70:(e)-4-(环戊氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-5)的制备
[0262][0263]
参照化合物8c-1的合成,以化合物7t-5(518mg,1.27mmol)为原料得到化合物8t-5,黄色固体260mg,收率为43%;1h nmr(300mhz,cdcl3)δ11.68(s,1h),7.80(d,j=15.9hz,1h),7.65
–
7.54(m,4h),6.74(d,j=15.9hz,1h),6.59(s,1h),5.24(dd,j=11.8,5.8hz,1h),5.00
–
4.90(m,1h),3.93(s,3h),3.37(d,j=7.1hz,2h),2.00
–
1.59(m,14h).
13
c nmr(75mhz,cdcl3)δ171.9,161.9,160.2,141.2,139.4 133.6,131.7,129.4(q,2j
c-f
=32.3hz),
128.5,126.8,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=270.2hz),122.2,118.0,105.2,104.1,79.7,52.4,33.2,26.0,24.1,22.5,18.0;esi-lrms m/z:472.9[m-h]-.
[0264]
实施例71:(e)-4-(环己氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-6)的制备
[0265][0266]
参照化合物8c-1的合成,以化合物7t-6(385mg,0.92mmol)为原料得到化合物8t-6,无色油状液体152mg,收率为34%;1h nmr(400mhz,cdcl3)δ11.68(s,1h),7.79(d,j=15.9hz,1h),7.61(q,j=8.2hz,4h),6.72(d,j=15.9hz,1h),6.57(s,1h),5.27(m,1h),4.47(m,1h),3.93(s,3h),3.41(d,j=6.9hz,3h),1.99(m,4h),1.81(s,3h),1.69(s,3h),1.67
–
1.40(m,6h).
13
c nmr(101mhz,cdcl3)δ171.8,162.1,160.0,141.2,139.4,133.6,131.6,128.5,126.8,129.5(q,2j
c-f
=33.3hz),125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=276.7hz),122.4,118.4,105.2,104.1,75.3,52.4,31.9,26.0,25.7,23.5,22.5,18.1;esi-lrms m/z:486.9[m-h]-.
[0267]
实施例72:(e)-4-(环庚氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-7)的制备
[0268][0269]
参照化合物8c-1的合成,以化合物7t-7(545mg,1.25mmol)为原料得到化合物8t-7,黄色固体265mg,收率为42%;1h nmr(300mhz,cdcl3)δ11.68(s,1h),7.80(d,j=15.9hz,1h),7.66
–
7.54(m,4h),6.72(d,j=15.9hz,1h),6.53(s,1h),5.24(t,j=7.1hz,1h),4.62(t,j=9.7hz,1h),3.93(s,3h),3.39(d,j=7.1hz,2h),2.08
–
1.47(m,18h).
13
c nmr(75mhz,cdcl3)δ171.8,162.1,160.0,141.2,139.4,133.6,131.7,129.5(q,2j
c-f
=32.3hz),128.5,126.8,125.8(q,3j
c-f
=3.8hz),123.8(q,1j
c-f
=270.2hz),122.3,118.3,105.2,104.0,77.9,52.4,34.0,28.7,26.0,23.1,22.5,18.1;esi-lrms m/z:501.0[m-h]-.
[0270]
实施例73:(e)-4-(苄氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-8)的制备
[0271][0272]
参照化合物8c-1的合成,以化合物7t-8(501mg,1.17mmol)为原料得到化合物8t-8,白色固体255mg,收率为44%;1h nmr(400mhz,cdcl3)δ11.72(s,1h),7.80(d,j=16.0hz,1h),7.61(dd,j=23.1,8.3hz,4h),7.41(m,5h),6.70(d,j=16.0hz,1h),6.67(s,1h),5.28(t,j=7.0hz,1h),5.22(s,2h),3.94(s,3h),3.48(d,j=7.0hz,2h),1.74(s,3h),1.70(s,
3h).
13
c nmr(101mhz,cdcl3)δ171.8,161.8,160.6,141.1,139.6,136.7,133.3,132.0,129.5(q,2j
c-f
=32.3hz),128.8,128.7,128.2,127.4,126.8,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=273.7hz),122.1,117.8,104.9,104.5,70.3,52.4,25.9,22.5,18.0;esi-lrms m/z:494.8[m-h]-.
[0273]
实施例74:(e)-4-(仲丁氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-9)的制备
[0274][0275]
参照化合物8c-1的合成,以化合物7t-9(395mg,1.00mmol)为原料得到化合物8t-9,黄色油状液体108mg,收率为23%;1h nmr(400mhz,cdcl3)δ11.69(s,1h),7.80(d,j=15.9hz,1h),7.61(q,j=8.6hz,4h),6.74(d,j=15.9hz,1h),6.58(s,1h),5.25(t,j=7.2hz,1h),4.53(dt,j=12.0,6.0hz,1h),3.91(s,3h),3.40(d,j=7.2hz,2h),1.81(s,3h),1.69(s,3h),1.35(d,j=6.0hz,3h),1.01(t,j=7.4hz,3h).
13
c nmr(101mhz,cdcl3)δ171.8,162.1,160.3,141.2,139.5,133.6,131.6,129.5(q,2j
c-f
=31.3hz),128.5,126.8,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.3,118.2,104.9,104.1,75.1,52.4,29.4,25.9,22.5,19.5,18.0,9.8;esi-lrms m/z:460.9[m-h]-.
[0276]
实施例75:(e)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(戊-3-基氧基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-10)的制备
[0277][0278]
参照化合物8c-1的合成,以化合物7t-10(411mg,1.01mmol)为原料得到化合物8t-10,白色粉末173mg,收率为36%;1h nmr(400mhz,cdcl3)δ11.68(s,1h),7.80(d,j=15.9hz,1h),7.61(q,j=8.5hz,4h),6.73(d,j=15.9hz,1h),6.56(s,1h),5.24(t,j=7.1hz,1h),4.36(p,j=5.6hz,1h),3.93(s,3h),3.40(d,j=7.2hz,2h),1.80(s,3h),1.78
–
1.71(m,4h),1.68(s,3h),0.98(t,j=7.4hz,6h).
13
c nmr(101mhz,cdcl3)δ171.8,162.1,160.7,141.3,139.4,133.7,131.6,129.5(q,2j
c-f
=32.3hz),128.4,126.8,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.4,118.1,104.8,104.0,79.9,52.4,26.3,25.9,22.5,18.0,9.6;esi-lrms m/z:477.3[m h]
.
[0279]
实施例76:(e)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(戊-3-基氧基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-11)的制备
[0280]
[0281]
参照化合物8c-1的合成,以化合物7t-11(464mg,1.10mmol)为原料得到化合物8t-11,黄色油状液体166mg,收率为30.8%;1h nmr(300mhz,cdcl3)δ11.68(s,1h),7.80(d,j=15.9hz,1h),7.63
–
7.56(m,4h),6.73(d,j=15.9hz,1h),6.56(s,1h),5.23(t,j=7.0hz,1h),4.42(p,j=2.0hz,1h),3.93(s,3h),3.40(d,j=7.1hz,2h),1.80(s,3h),1.79
–
1.70(m,6h),1.68(s,3h),0.96(q,j=7.2hz,7h).
13
c nmr(75mhz,cdcl3)δ171.8,162.1,160.7,141.2,139.4,133.7,131.6,129.5(q,2j
c-f
=32.3hz),128.4,126.8,126.2,125.8(q,3j
c-f
=3.8hz),124.3(q,1j
c-f
=272.7hz),122.3,118.0,104.7,104.0,78.5,52.4,35.8,26.8,25.9,22.5,18.7,18.0,14.4,9.5;esi-lrms m/z:491.3[m h]
.
[0282]
实施例77:(e)-4-(2-氟乙氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-12)的制备
[0283][0284]
参照化合物8c-1的合成,以化合物7t-12(444mg,1.16mmol)为原料得到化合物8t-12,白色固体146mg,收率为28%;1h nmr(400mhz,cdcl3)δ11.69(s,1h),7.78(d,j=16.0hz,1h),7.60(dd,j=19.3,8.4hz,4h),6.76(d,j=16.0hz,1h),6.57(s,1h),5.26(t,j=7.2hz,1h),4.79(m,2h),4.46
–
4.23(m,2h),3.94(s,3h),3.43(d,j=7.2hz,2h),1.81(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ171.7,161.9,160.3,141.1,139.7,133.2,132.1,129.6(q,2j
c-f
=32.3hz),126.8,125.9(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=273.7hz),121.8,117.9,105.2,103.9,81.8(d,1j
c-f
=172.7hz),67.7(d,2j
c-f
=21.2hz),52.5,25.9,22.4,17.9;esi-lrms m/z:450.8[m-h]-.
[0285]
实施例78:(e)-4-(2,2-二氟乙氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-13)的制备
[0286][0287]
参照化合物8c-1的合成,以化合物7t-13(436mg,1.08mmol)为原料得到化合物8t-13,白色固体204mg,收率为40%;1h nmr(400mhz,cdcl3)δ11.71(s,1h),7.78(d,j=16.0hz,1h),7.61(dd,j=18.6,8.4hz,4h),6.77(d,j=16.0hz,1h),6.53(s,1h),6.14(tt,j=55.0,4.1hz,1h),5.23(t,j=7.1hz,1h),4.30(td,j=12.9,4.1hz,2h),3.95(s,3h),3.41(d,j=7.2hz,2h),1.80(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ171.7,162.0,159.4,140.9,139.8,132.8,132.3,129.7(q,2j
c-f
=33.3hz),129.1,126.8,125.9(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),121.6,118.1,113.6(t,1j
c-f
=242.4hz),105.8,103.7,67.6(t,2j
c-f
=30.3hz),52.6,25.9,22.4,17.8;esi-lrms m/z:468.8[m-h]-.
[0288]
实施例79:(e)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(2,2,2-三氟乙氧基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-14)的制备
[0289][0290]
参照化合物8c-1的合成,以化合物7t-14(473mg,1.13mmol)为原料得到化合物8t-14,白色固体165mg,收率为30%;1h nmr(400mhz,cdcl3)δ11.71(s,1h),7.77(d,j=16.0hz,1h),7.61(dd,j=19.6,8.3hz,4h),6.76(d,j=16.0hz,1h),6.51(s,1h),5.23(t,j=6.7hz,1h),4.47(q,j=7.9hz,2h),3.96(s,3h),3.43(d,j=7.1hz,2h),1.79(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ171.6,162.1,158.9,140.9,139.9,132.7,132.5,129.8(q,2j
c-f
=32.3hz),129.2,126.9,125.9(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=272.7hz),123.1(q,1j
c-f
=278.8hz),121.3,118.6,106.2,103.8,66.0(q,1j
c-f
=36.4hz),52.6,25.9,22.4,17.8;esi-lrms m/z:486.8[m-h]-.
[0291]
实施例80:(e)-4-(3-氟丙氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-15)的制备
[0292][0293]
参照化合物8c-1的合成,以化合物7t-15(483mg,1.21mmol)为原料得到化合物8t-15,白色晶体198mg,收率为35%;1h nmr(400mhz,cdcl3)δ11.71(s,1h),7.80(d,j=26.6hz,1h),7.63(q,j=8.5hz,4h),6.80(d,j=16.0hz,1h),6.63(s,1h),5.24(t,j=7.0hz,1h),4.71(dt,j=47.0,6.0hz,2h),4.26(t,j=6.0hz,2h),3.42(d,j=7.0hz,2h),2.26(dp,j=26.0,6.0hz,2h),1.82(s,3h),1.72(s,3h).
13
c nmr(101mhz,cdcl3)δ171.8,161.8,160.7,141.1,139.7,133.2,131.8,129.6(q,2j
c-f
=32.3hz),128.7,126.8,125.9(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.2,117.5,104.9,103.8,80.7(d,1j
c-f
=163.6hz),64.1(d,3j
c-f
=5.1hz),52.4,30.6(d,2j
c-f
=20.2hz),25.9,22.4,18.0;esi-lrms m/z:464.8[m-h]-.
[0294]
实施例81:(e)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)-4-(3,3,3-三氟丙氧基)苯甲酸甲酯(8t-16)的制备
[0295][0296]
参照化合物8c-1的合成,以化合物7t-10(426mg,0.98mmol)为原料得到化合物8t-16,黄色晶体197mg,收率为40%;1h nmr(400mhz,cdcl3)δ11.70(s,1h),7.79(d,j=16.0hz,1h),7.61(q,j=8.5hz,4h),6.78(d,j=16.0hz,1h),6.56(s,1h),5.30
–
5.14(m,1h),4.33
(t,j=6.5hz,2h),3.95(s,3h),3.40(d,j=7.1hz,2h),2.70(qt,j=10.5,6.5hz,2h),1.80(s,3h),1.70(s,3h).
13
c nmr(101mhz,cdcl3)δ171.7,161.9,159.9,141.0,139.8,133.1,132.0,129.7(q,2j
c-f
=32.3hz),128.9,126.8,125.9(q,1j
c-f
=277.8hz),125.9(q,3j
c-f
=3.0hz),124.4(q,1j
c-f
=272.7hz),121.9,117.8,105.3,103.5,61.4(q,3j
c-f
=3.0hz),52.5,34.3(q,2j
c-f
=29.3hz),25.9,22.3,17.9;esi-lrms m/z:500.7[m-h]-.
[0297]
实施例82:(e)-4-(4-氟丁氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-17)的制备
[0298][0299]
参照化合物8c-1的合成,以化合物7t-17(509mg,1.23mmol)为原料得到化合物8t-17,白色晶体237mg,收率为40%;1h nmr(400mhz,cdcl3)δ11.69(s,1h),7.79(d,j=16.0hz,1h),7.61(q,j=8.4hz,4h),6.77(d,j=16.0hz,1h),6.58(s,1h),5.23(t,j=7.0hz,1h),4.55(dt,j=11.4,5.5hz,2h),4.15(t,j=5.8hz,2h),3.94(s,3h),3.40(d,j=7.0hz,2h),2.13
–
1.84(m,4h),1.80(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ171.8,161.8,160.8,141.1,139.7,133.3,131.8,129.5(q,2j
c-f
=32.3hz),128.6,126.8,125.8(q,3j
c-f
=4.0hz),124.0(q,1j
c-f
=272.7hz),122.2,117.4,104.7,103.8,83.8(d,1j
c-f
=165.6hz),67.7,52.4,27.2(d,2j
c-f
=20.2hz),25.9,25.5(d,3j
c-f
=4.0hz),22.4,18.0;esi-lrms m/z:478.8[m-h]-.
[0300]
实施例83:(e)-4-(4,4,4-三氟丁氧基)-3-(3-甲基丁-2-烯-1-基)-2-羟基-6-(4-(三氟甲基)苯乙烯基)苯甲酸甲酯(8t-18)的制备
[0301][0302]
参照化合物8c-1的合成,以化合物7t-18(512mg,1.14mmol)为原料得到化合物8t-18,白色固体271mg,收率为46%;1h nmr(400mhz,cdcl3)δ11.71(s,1h),7.79(d,j=16.0hz,1h),7.61(q,j=8.4hz,4h),6.77(d,j=16.0hz,1h),6.56(s,1h),5.20(t,j=6.4hz,1h),4.16(t,j=5.9hz,2h),3.94(s,3h),3.41(d,j=6.9hz,2h),2.43
–
2.27(m,2h),2.12(m,2h),1.80(s,3h),1.70(s,3h).
13
c nmr(101mhz,cdcl3)δ171.8,161.8,160.5,141.1,139.8,133.2,132.0,129.6(q,2j
c-f
=32.3hz),128.8,126.8,125.7(q,1j
c-f
=278.8hz),125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.2,117.5,105.0,103.7,66.5,52.5,30.8(q,2j
c-f
=29.3hz),25.8,22.4(q,3j
c-f
=3.0hz),17.9;esi-lrms m/z:514.8[m-h]-.
[0303]
实施例84:(e)-4-乙氧基-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(c1)的制备
[0304][0305]
在伴有回流装置的50ml圆底烧瓶中,以10ml乙醇将化合物8c-1(152mg,0.40mmol)溶解,搅拌,将氢氧化钠(80mg,1.98mmol)溶解在2ml水中,随后加进反应液中,80℃搅拌。tlc监测反应进度,待反应结束,停止加热,以2m hcl调节反应液体系ph为2-3,析出大量固体,减压过滤,经重结晶得到黄色粉末116mg,收率为78%,纯度为95.05%,熔点为165.3-166.1℃;1h nmr(300mhz,cdcl3)δ11.52(s,1h),7.75(d,j=15.9hz,1h),7.56
–
7.41(m,2h),7.15
–
7.00(m,2h),6.75(d,j=15.9hz,1h),6.61(s,1h),5.32
–
5.15(m,1h),4.18(q,j=6.9hz,2h),3.39(d,j=7.3hz,2h),1.80(s,3h),1.69(s,3h),1.47(t,j=6.9hz,3h).
13
c nmr(75mhz,cdcl3)δ176.2,162.7,162.6(d,1j
c-f
=247.5hz),162.0,141.8,133.6(d,4j
c-f
=3.8hz),130.3(d,5j
c-f
=1.5hz),129.6,128.5(d,3j
c-f
=8.3hz),122.0,117.0,116.0,115.8(d,2j
c-f
=21.8hz),104.3,102.9,64.2,26.0,22.3,18.0,15.0;esi-lrms m/z:368.9[m-h]-;esi-hrms m/z:397.1491[m-h]-,calcd for c
22h22
o4f 369.1508.
[0306]
实施例85:(e)-6-(4-氟苯乙烯基)-2-羟基-4-异丙氧基-3-(3-甲基丁-2-烯-1-基)苯甲酸(c2)的制备
[0307][0308]
参照化合物c1的合成,以化合物8c-2(185mg,0.46mmol)为原料得到化合物c2,黄色粉末143mg,收率为81%,纯度为97.77%,熔点为154.7-155.3℃;1h nmr(300mhz,cdcl3)δ11.54(s,1h),7.77(d,j=15.9hz,1h),7.58
–
7.44(m,2h),7.09(t,j=8.7hz,2h),6.75(d,j=15.9hz,1h),6.64(s,1h),5.34
–
5.19(m,1h),4.78(hept,j=6.0hz,1h),3.40(d,j=7.2hz,2h),1.83(s,3h),1.71(s,3h),1.42(d,j=6.0hz,6h).
13
c nmr(75mhz,cdcl3)δ176.5,163.0,162.6(d,1j
c-f
=245.3hz),161.1,141.7,133.6(d,4j
c-f
=3.8hz),131.7,130.5(d,5j
c-f
=2.3hz),129.5,128.5(d,3j
c-f
=7.5hz),122.1,117.8,115.7(d,2j
c-f
=21.8hz),105.3,102.5,70.4,26.0,22.3,18.1;esi-lrms m/z:382.9[m-h]-;esi-hrms m/z:383.1653[m-h]-,calcd for c
23h24
o4f 383.1664.
[0309]
实施例86:(e)-4-(环丙基甲氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(c3)的制备
[0310][0311]
参照化合物c1的合成,以化合物8c-3(223mg,0.54mmol)为原料得到化合物c3,白色固体179mg,收率为83%,纯度为97.59%,熔点为163.0-163.9℃;1h nmr(400mhz,cdcl3)δ11.51(s,1h),7.75(d,j=15.9hz,1h),7.54
–
7.42(m,2h),7.07(t,j=8.7hz,2h),6.75(d,j
=15.9hz,1h),6.58(s,1h),5.28(t,j=7.2hz,1h),3.96(d,j=6.8hz,2h),3.42(d,j=7.2hz,2h),1.83(s,3h),1.70(s,3h),1.39
–
1.17(m,1h),0.70
–
0.64(q,j=5.0hz,2h),0.40(q,j=5.0hz,2h).
13
c nmr(101mhz,cdcl3)δ176.2,162.7,162.7(d,1j
c-f
=246.0hz),162.1,141.8,133.7(d,4j
c-f
=3.0hz),131.9,130.4(d,5j
c-f
=2.0hz),129.7,128.5(d,3j
c-f
=8.1hz),122.1,117.2,115.8(d,2j
c-f
=22.2hz),104.4,103.0,73.1,26.0,22.4,18.0,10.4,3.3;esi-lrms m/z:394.9[m-h]-;esi-hrms m/z:395,1659[m-h]-,calcd for c
24h23
o4f4395.1664.
[0312]
实施例87:(e)-4-(环戊氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(c4)的制备
[0313][0314]
参照化合物c1的合成,以化合物8c-4(112mg,0.26mmol)为原料得到化合物c4,白色粉末85mg,收率为78%,纯度为97.41%,熔点为142.7-143.4℃;1h nmr(400mhz,cdcl3)δ11.51(s,1h),7.76(d,j=15.9hz,1h),7.58
–
7.40(m,2h),7.08(t,j=8.7hz,2h),6.74(d,j=15.9hz,1h),6.63(s,1h),5.23(t,j=7.2hz,1h),5.07
–
4.89(m,1h),3.36(d,j=7.1hz,2h),2.08
–
1.59(m,8h),1.80(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ176.2,162.8,162.3(d,1j
c-f
=248.5hz),161.2,141.6,133.7(d,4j
c-f
=3.0hz),131.7,130.5(d,5j
c-f
=2.0hz),129.6,128.5(d,3j
c-f
=8.1hz),122.2,117.6,115.9(d,2j
c-f
=21.2hz),105.5,102.6,79.9,33.2,26.0,24.2,22.4,18.0;esi-lrms m/z:408.9[m-h]-;esi-hrms m/z:409.1813[m-h]-,calcd for c
25h26
o4f 409.1821.
[0315]
实施例88:(e)-4-(苄氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(c5)的制备
[0316][0317]
参照化合物c1的合成,以化合物8c-5(235mg,0.53mmol)为原料得到化合物c5,白色固体178mg,收率为78%,纯度为96.99%,熔点为178.0-178.6℃;1h nmr(400mhz,dmso-d6)δ12.76(s,1h),7.80(d,j=16.1hz,1h),7.58-7.55(m,2h),7.49-7.32(m,5h),7.22(t,j=8.8hz,2h),6.94(d,j=16.1hz,1h),6.88(s,1h),5.28(s,2h),5.15(t,j=7.0hz,1h),3.30(d,j=7.0hz,2h),1.62(s,3h),1.61(s,3h).
13
c nmr(101mhz,dmso-d6)δ173.3,161.7(d,1j
c-f
=245.4hz),160.9,159.7,139.6,136.9,133.9(d,4j
c-f
=3.0hz),130.6,129.8(d,5j
c-f
=2.0hz),128.8,128.4(d,3j
c-f
=8.1hz),128.3,127.9,127.5,122.1,115.6(d,2j
c-f
=21.2hz),105.1,103.5,69.6,25.5,21.9,17.6;esi-lrms m/z:430.8[m-h]-;esi-hrms m/z:431.1647[m-h]-,calcd for c
27h24
o4f 431.1664.
[0318]
实施例89:(e)-4-(环丁氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(y1)的制备
[0319][0320]
参照化合物c1的合成,以化合物8y-1(121mg,0.29rmmol)为原料得到化合物y1,白色晶体61mg,收率为52%,纯度为97.34%,熔点为167.0-168.0℃;1h nmr(300mhz,cdcl3)δ11.51(s,1h),7.74(d,j=15.9hz,1h),7.54
–
7.43(m,2h),7.07(t,j=8.7hz,2h),6.72(d,j=15.9hz,1h),6.47(s,1h),5.35
–
5.05(m,1h),4.80(h,j=6.0hz,1h),3.38(d,j=7.1hz,2h),2.58
–
2.41(m,2h),2.32
–
2.11(m,2h),1.97
–
170(m,8h).
13
c nmr(75mhz,cdcl3)δ176.1,162.8,162.7(d,1j
c-f
=247.5hz),161.0,160.7,141.7,133.7(d,4j
c-f
=3.0hz),131.9,130.4(d,5j
c-f
=2.3hz),129.6,128.5(d,3j
c-f
=7.5hz),122.0,117.1,115.9(d,2j
c-f
=22.5hz),105.1,102.9,72.0,30.9,26.0,22.4,18.0,13.5;esi-lrms m/z:394.9[m-h]-;esi-hrms m/z:395.1642[m-h]-,calcd for c
24h24
o4f 395.1664.
[0321]
实施例90:(e)-4-(环己氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(y2)的制备
[0322][0323]
参照化合物c1的合成,以化合物8y-2(156mg,0.36mmol)为原料得到化合物y2,黄色晶体109mg,收率为72%,纯度为98.60%,熔点为159.8-160.4℃;1h nmr(300mhz,cdcl3)δ11.54(s,1h),7.75(d,j=15.9hz,1h),7.56
–
7.45(m,2h),7.08(t,j=8.7hz,2h),6.72(d,j=15.9hz,1h),6.61(s,1h),5.37
–
5.15(m,1h),4.64
–
4.39(m,1h),3.40(d,j=7.1hz,2h),2.06
–
1.81(m,7h),1.69(s,3h),1.65
–
1.36(m,6h).
13
c nmr(75mhz,cdcl3)δ176.2,163.1,162.7(d,1j
c-f
=247.5hz),161.0,141.6,133.7(d,4j
c-f
=3.8hz),131.7,130.5(d,5j
c-f
=2.3hz),129.5,128.5(d,3j
c-f
=7.5hz),122.3,117.8,115.9(d,2j
c-f
=22.5hz),105.4,102.5,75.3,31.9,26.0,25.7,23.5,22.5,18.1;esi-lrms m/z:422.9[m-h];esi-hrms m/z:423.1980[m-h]-,calcd for c
26h28
o4f 423.1977.
[0324]
实施例91:(e)-4-(环庚氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(y3)的制备
[0325][0326]
参照化合物c1的合成,以化合物8y-3(212mg,0.47mmol)为原料得到化合物y3,黄色晶体146mg,收率为71%,纯度为97.58%,熔点为166.7-167.3℃;1h nmr(300mhz,cdcl3)δ11.53(s,1h),7.77(d,j=15.9hz,1h),7.58
–
7.44(m,2h),7.08(t,j=8.7hz,2h),6.73(d,j=15.9hz,1h),6.58(s,1h),5.24(t,j=7.1hz,1h),4.77
–
4.53(m,1h),3.39(d,j=7.1hz,2h),2.17
–
1.43(m,12h),1.81(s,3h),1.69(s,3h).
13
c nmr(75mhz,cdcl3)δ176.3,163.0,
162.7(d,1j
c-f
=245.3hz),161.0,141.6,133.7(d,4j
c-f
=3.0hz),131.7,130.6(d,5j
c-f
=2.3hz),129.5,128.5(d,3j
c-f
=8.3hz),122.2,117.8,115.9(d,2j
c-f
=21.8hz),105.5,102.4,78.0,34.0,28.7,26.0,23.1,22.5,18.1;esi-lrms m/z:436.9[m-h]-;esi-hrms m/z:437.2113[m-h]-,calcd for c
27h30
o4f 437.2134.
[0327]
实施例92:(e)-4-(仲丁氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(y4)的制备
[0328][0329]
参照化合物c1的合成,以化合物8y-4(204mg,0.49mmol)为原料得到化合物y4,白色晶体144mg,产率73%,纯度为98.19%,熔点为135.2-135.9℃;1h nmr(300mhz,cdcl3)δ11.54(s,1h),7.76(d,j=15.9hz,1h),7.55
–
7.44(m,2h),7.08(t,j=8.7hz,2h),6.74(d,j=15.9hz,1h),6.61(s,1h),5.44
–
4.92(m,1h),4.54(h,j=6.0hz,1h),3.38(d,j=7.1hz,2h),1.87
–
1.65(m,8h),1.36(d,j=6.1hz,3h),1.01(t,j=7.4hz,3h).
13
c nmr(75mhz,cdcl3)δ176.1,163.0,162.7(d,1j
c-f
=247.5hz),161.3,141.7,133.7(d,4j
c-f
=3.0hz),131.7,130.5(d,5j
c-f
=3.0hz),129.5,128.5(d,3j
c-f
=7.5hz),122.2,117.7,115.9(d,2j
c-f
=22.5hz),105.2,102.5,75.2,29.4,26.0,22.4,19.6,18.0,9.8;esi-lrms m/z:396.9[m-h]-;esi-hrms m/z:397.1800[m-h]-,calcd for c
24h26
o4f 397.1821.
[0330]
实施例93:(e)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(戊基-3-基氧基)苯甲酸(y5)的制备
[0331][0332]
参照化合物c1的合成,以化合物8y-5(176mg,0.41mmol)为原料得到化合物y5,白色粉末129mg,收率为76%,纯度为97.85%,熔点为117.0-117.6℃;1h nmr(300mhz,cdcl3)δ11.54(s,1h),7.76(d,j=15.9hz,1h),7.56
–
7.44(m,2h),7.12
–
7.02(m,2h),6.73(d,j=15.9hz,1h),6.59(s,1h),5.32
–
5.07(m,1h),4.39(p,j=6.0hz 1h),3.39(d,j=7.1hz,2h),1.80(s,3h),1.78-1.71(qd,j=7.5,6.0hz,4h),1.68(s,3h),0.98(t,j=7.4hz,6h).
13
c nmr(75mhz,cdcl3)δ176.1,163.0,162.7(d,1j
c-f
=247.5hz),161.7,141.6,133.7(d,4j
c-f
=3.8hz),131.6,130.6(d,5j
c-f
=2.3hz),129.5,128.5(d,3j
c-f
=7.5hz),122.3,117.6,115.9(d,2j
c-f
=22.5hz),105.1,102.4,79.9,26.3,26.0,22.5,18.0,9.6;esi-lrms m/z:410.9[m-h]-;esi-hrms m/z:411.1953[m-h]-,calcd for c
24h28
o4f 411.1977.
[0333]
实施例94:(e)-6-(4-氟苯乙烯基)-4-(己-3-基氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(y6)的制备
[0334][0335]
参照化合物c1的合成,以化合物8y-6(191mg,0.43mmol)为原料得到化合物y6,白色固体142mg,收率为77%,纯度为98.20%,熔点为92.8-93.5℃;1h nmr(300mhz,cdcl3)δ11.54(s,1h),7.75(d,j=15.9hz,1h),7.50(m,2h),7.08(t,j=8.7hz,2h),6.73(d,j=15.9hz,1h),6.60(s,1h),5.22(t,j=6.6hz,1h),4.45(p,j=6.0hz,1h),3.38(d,j=7.1hz,2h),1.80(s,3h),1.68(s,3h),1.84
–
1.59(m,4h),1.52
–
1.33(m,2h),0.97(q,j=7.3hz,6h).
13
c nmr(75mhz,cdcl3)δ176.0,163.0,162.7(d,1j
c-f
=247.5hz),161.8,141.7,133.7(d,4j
c-f
=3.8hz),131.6,130.6(d,5j
c-f
=2.3hz),129.5,128.5(d,3j
c-f
=7.5hz),122.3,117.6,115.9(d,2j
c-f
=21.0hz),105.0,102.4,78.6,35.8,26.8,25.9,22.4,18.7,18.0,14.4,9.5;esi-lrms m/z:424.9[m-h]-;esi-hrms m/z:425.2159[m-h]-,calcd for c
26h30
o4f 425.2134.
[0336]
实施例95:(e)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-((四氢-2h-吡喃-4-基)氧基)苯甲酸(y7)的制备
[0337][0338]
参照化合物c1的合成,以化合物8y-7(112mg,0.25mmol)为原料得到化合物y7,白色晶体56mg,收率为56%,纯度为95.48%,熔点为148.5-149.4℃;1h nmr(400mhz,cdcl3)δ11.63(s,1h),7.74(d,j=15.9hz,1h),7.56
–
7.40(m,2h),7.07(t,j=8.7hz,2h),6.71(d,j=15.9hz,1h),6.59(s,1h),5.23(t,j=6.5hz,1h),4.74(dq,j=10.6,3.6hz,1h),4.06
–
3.94(m,2h),3.76
–
3.60(m,2h),3.41(d,j=7.0hz,2h),2.09(m,2h),1.88(m,2h),1.80(s,3h),1.70(s,3h).
13
c nmr(101mhz,cdcl3)δ175.6,163.1,162.6(d,1j
c-f
=248.5hz),160.1,141.6,133.6(d,4j
c-f
=4.0hz),131.9,130.4(d,5j
c-f
=3.0hz),129.6,128.5(d,3j
c-f
=8.1hz),122.1,118.0,115.8(d,2j
c-f
=21.2hz),105.1,103.2,71.3,64.9,31.8,25.9,22.5,18.1;esi-lrms m/z:424.9[m-h]-;esi-hrms m/z:425.1767[m-h]-,calcd for c
25h26
o5f425.11770.
[0339]
实施例96:(e)-4-(2-氟乙氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(y8)的制备
[0340][0341]
参照化合物c1的合成,以化合物8y-8(111mg,0.28mmol)为原料得到化合物y8,白色粉末88mg,收率为82%,纯度为95.15%,熔点为165.6-166.4℃;1h nmr(300mhz,cdcl3)δ
11.53(s,1h),7.74(d,j=15.9hz,1h),7.48(dd,j=8.6,5.4hz,2h),7.07(t,j=8.6hz,2h),6.75(d,j=15.9hz,1h),6.59(s,1h),5.24(t,j=7.2hz,1h),5.16
–
5.15(m,1h),4.92
–
4.66(m,2h),4.44
–
4.26(m,2h),3.41(d,j=7.1hz,2h),1.81(s,3h),1.69(s,3h).
13
c nmr(75mhz,cdcl3)δ175.9,162.7,161.8(d,1j
c-f
=246.8hz),161.1,141.8,133.4(d,4j
c-f
=3.0hz),132.0,130.0(d,5j
c-f
=2.3hz),129.8,128.4(d,3j
c-f
=8.3hz),121.6,117.3,115.7(d,2j
c-f
=21.8hz),104.0,103.5,81.6(d,1j
c-f
=171.0hz),67.5(d,2j
c-f
=20.3hz),25.9,22.2,17.8;esi-lrms m/z:386.9[m-h]-;esi-hrms m/z:387.1413[m-h]-,calcd for c
22h21
o4f2387.1405.
[0342]
实施例97:(e)-4-(2,2-二氟乙氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(y9)的制备
[0343][0344]
参照化合物c1的合成,以化合物8y-9(161mg,0.38mmol)为原料得到化合物y9,白色晶体125mg,收率为80%,纯度为95.21%,熔点为163.6-164.3℃;1h nmr(400mhz,meod)δ7.79(d,j=16.1hz,1h),7.49(dd,j=8.4,5.6hz,2h),7.03(t,j=8.6hz,2h),6.84(d,j=16.0hz,1h),6.65(s,1h),6.17(tt,j=54.9,3.6hz,1h),5.18(t,j=6.8hz,1h),4.32(td,j=13.7,3.7hz,2h),3.32(d,j=7.2hz,2h),1.74(s,3h),1.63(s,3h).
13
c nmr(101mhz,meod)δ174.7,163.8(d,1j
c-f
=246.4hz),163.2,160.7,142.1,135.5(d,4j
c-f
=3.0hz),132.1(d,5j
c-f
=2.0hz),131.2,130.2,129.4(d,3j
c-f
=8.1hz),123.2,117.8,116.4(d,2j
c-f
=21.2hz),115.3(t,1j
c-f
=240.4hz),106.5,103.9,68.4(t,2j
c-f
=27.3hz),26.0,23.0,17.9;esi-lrms m/z:404.8[m-h]-;esi-hrms m/z:405.1300[m-h]-,calcd for c
22h20
o4f
3 405.1319.
[0345]
实施例98:(e)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(2,2,2-三氟乙氧基)苯甲酸(y10)的制备
[0346][0347]
参照化合物c1的合成,以化合物8y-10(116mg,0.26mmol)为原料得到化合物y10,白色晶体71mg,收率为63%,纯度为97.67%,熔点为176.5-177.3℃;1h nmr(400mhz,cdcl3)δ11.54(s,1h),7.72(d,j=15.9hz,1h),7.55
–
7.42(m,2h),7.08(m,2h),6.76(d,j=15.9hz,1h),6.54(s,1h),5.22(m,1h),4.48(q,j=7.9hz,2h),3.42(d,j=7.1hz,2h),1.78(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ175.6,163.0,162.8(d,1j
c-f
=249.5hz),159.8,142.0,133.4(d,4j
c-f
=3.0hz),132.6,130.4,129.7(d,5j
c-f
=3.0hz),128.6(d,3j
c-f
=8.1hz),123.3(d,1j
c-f
=278.8hz),121.3,118.2,116.0(d,2j
c-f
=22.2hz),104.6,104.0,66.0(d,2j
c-f
=36.4hz),25.9,22.4,17.8;esi-lrms m/z:422.8[m-h]-;esi-hrms m/z:
423.1219[m-h]-,calcd for c
22h19
o4f
4 423.1225.
[0348]
实施例99:(e)-4-(3-氟丙氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(y11)的制备
[0349][0350]
参照化合物c1的合成,以化合物8y-11(179mg,0.42mmol)为原料得到化合物y11,白色固体133mg,收率为77%,纯度为98.84%,熔点为150.6-151.3℃;1h nmr(400mhz,cdcl3)δ11.53(s,1h),7.75(d,j=15.9hz,1h),7.49(m,2h),7.07(m,2h),6.78(d,j=15.9hz,1h),6.64(s,1h),5.21(t,j=6.5hz,1h),4.68(dt,j=47.0,6.0hz,2h),4.26(t,j=6.0hz,2h),3.39(d,j=6.9hz,2h),2.35
–
2.14(dp,j=26.0,6.0hz,2h),1.79(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ175.9,162.7,162.7(d,1j
c-f
=248.5hz),61.6,142.0,133.6(d,4j
c-f
=4.0hz),131.9,130.2(d,5j
c-f
=2.0hz),129.9,128.5(d,3j
c-f
=8.1hz),122.1,117.1,115.8(d,2j
c-f
=22.2hz),104.1,103.3,81.5,79.8,64.1(d,3j
c-f
=5.1hz),64.1,30.6(d,2j
c-f
=20.2hz),25.9,22.3,18.0;esi-lrms m/z:400.8[m-h]-;esi-hrms m/z:401.1578[m-h]-,calcd for c
23h23
o4f
2 401.1570.
[0351]
实施例100:(e)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(3,3,3-三氟丙氧基)苯甲酸(y12)的制备
[0352][0353]
参照化合物c1的合成,以化合物8y-12(132mg,0.29mmol)为原料得到化合物y12,白色粉末90mg,收率为70%,纯度为95.23%,熔点为153.9-154.7℃;1h nmr(300mhz,cdcl3)δ11.55(s,1h),7.74(d,j=15.9hz,1h),7.56
–
7.40(m,2h),7.07(t,j=8.7hz,2h),6.77(d,j=15.9hz,1h),6.59(s,1h),5.20(t,j=6.1hz,1h),4.34(t,j=6.5hz,2h),3.38(d,j=7.1hz,2h),2.84
–
2.53(m,2h),1.79(s,3h),1.69(s,3h).
13
c nmr(75mhz,cdcl3)δ175.9,162.8,162.7(d,1j
c-f
=246.0hz),160.8,142.0,133.5(d,4j
c-f
=3.8hz),132.1,131.4,130.0(d,5j
c-f
=2.3hz),130.0,128.5(d,3j
c-f
=7.5hz),125.9(q,1j
c-f
=274.5hz),121.8,117.3,115.7(d,2j
c-f
=21.0hz),103.8,103.7,61.4(q,3j
c-f
=3.8hz),34.3(q,2j
c-f
=28.5hz),25.9,22.3,17.9;esi-lrms m/z:436.8[m-h]-;esi-hrms m/z:437.1358[m-h]-,calcd for c
23h21
o4f
4 437.1381.
[0354]
实施例101:(e)-4-(4-氟丁氧基)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)苯甲酸(y13)的制备
[0355][0356]
参照化合物c1的合成,以化合物8y-13(238mg,0.55mmol)为原料得到化合物y13,黄色固体121mg,收率为53%,纯度为97.99%,熔点为147.3-147.9℃;1h nmr(400mhz,cdcl3)δ11.52(s,1h),7.75(d,j=15.9hz,1h),7.55
–
7.43(m,2h),7.07(t,j=8.7hz,2h),6.77(d,j=15.9hz,1h),6.62(s,1h),5.28
–
5.08(m,1h),4.52(dd,j=26.7,20.8hz,1h),4.16(t,j=5.9hz,2h),3.39(d,j=7.0hz,2h),2.16
–
1.85(m,4h),1.79(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ176.1,162.7,162.7(d,1j
c-f
=248.5hz),161.8,141.9,133.6(d,4j
c-f
=4.0hz),131.9,130.3(d,5j
c-f
=2.0hz),129.8,128.5(d,3j
c-f
=8.1hz),122.1,117.0,115.8(d,2j
c-f
=21.2hz),104.1,103.1,83.8(d,1j
c-f
=166.7hz),67.8,31.7,27.3(d,2j
c-f
=20.2hz),25.9,25.5(d,3j
c-f
=5.1hz),22.3,18.0;esi-lrms m/z:414.9[m-h]-;esi-lrms m/z:414.9[m-h]-;esi-hrms m/z:415.1712[m-h]-,calcd for c
24h25
o4f
2 415.1726.
[0357]
实施例102:(e)-6-(4-氟苯乙烯基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(4,4,4-三氟丁氧基)苯甲酸(y14)的制备
[0358][0359]
参照化合物c1的合成,以化合物8y-14(213mg,0.46mmol)为原料得到化合物y14,白色固体147mg,收率为71%,纯度为96.46%,熔点为158.7-159.4℃;1h nmr(400mhz,cdcl3)δ11.53(s,1h),7.74(d,j=15.9hz,1h),7.49(dd,j=8.3,5.6hz,2h),7.07(t,j=8.6hz,2h),6.76(d,j=15.9hz,1h),6.59(s,1h),5.18(t,j=6.4hz,1h),4.17(t,j=5.7hz,2h),3.39(d,j=6.7hz,2h),2.43
–
2.24(m,2h),2.20
–
2.03(m,2h),1.79(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ176.0,162.7,162.7(d,1j
c-f
=248.5hz),161.4,142.0,133.5(d,4j
c-f
=3.0hz),132.1,130.1(d,5j
c-f
=130.1hz),129.9,128.5(d,3j
c-f
=8.1hz),124.4(q,1j
c-f
=276.7hz),122.1,117.1,115.9(d,2j
c-f
=248.5hz),104.0,103.4,66.6,30.8(q,2j
c-f
=29.3hz),25.9,22.3(q,3j
c-f
=3.0hz),17.9;esi-lrms m/z:450.8[m-h]-;esi-hrms m/z:451.1497[m-h]-,calcd for c
24h23
o4f
4 451.1538.
[0360]
实施例103:(e)-4-乙氧基-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t1)的制备
[0361]
[0362]
参照化合物c1的合成,以化合物8t-1(154mg,0.35mmol)为原料得到化合物t1,白色粉末84mg,收率为57%,纯度为95.19%,熔点为170.8-171.4℃;1h nmr(400mhz,cdcl3)δ11.49(s,1h),7.91(d,j=15.9hz,1h),7.71
–
7.53(m,4h),6.80(d,j=15.9hz,1h),6.62(s,1h),5.25(t,j=7.0hz,1h),4.18(q,j=6.9hz,2h),3.40(d,j=7.0hz,2h),1.81(s,3h),1.70(s,3h),1.48(t,j=6.9hz,3h).
13
c nmr(101mhz,cdcl3)δ176.2,162.8,162.0,141.3,140.9,133.1,132.0,129.6(q,2j
c-f
=32.3hz),129.3,127.0,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),121.9,117.5,104.5,103.0,64.2,26.0,22.4,18.0,14.9;esi-lrms m/z:418.8[m-h]-;esi-hrms m/z:419.1445[m-h]-,calcd for c
23h22
o4f
3 419.1476.
[0363]
实施例104:(e)-2-羟基-4-异丙氧基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t2)的制备
[0364][0365]
参照化合物c1的合成,以化合物8t-2(227mg,0.51mmol)为原料得到化合物t2,白色固体152mg,收率为70%,纯度为97.34%,熔点为160.6-161.4℃;1h nmr(400mhz,cdcl3)δ11.51(s,1h),7.93(d,j=15.9hz,1h),7.61(q,j=8.4hz,4h),6.78(d,j=15.9hz,1h),6.64(s,1h),5.25(t,j=7.1hz,1h),4.91
–
4.54(m,1h),3.39(d,j=7.1hz,2h),1.82(s,3h),1.70(s,3h),1.42(d,j=6.0hz,6h).
13
c nmr(101mhz,cdcl3)δ176.2,163.1,161.2,141.1,140.9,133.3,131.8,129.6(q,2j
c-f
=32.3hz),129.2,127.0,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=272.7hz),122.0,118.3,105.6,102.6,70.5,26.0,22.4,22.3,18.1;esi-lrms m/z:432.9[m-h]-;esi-hrms m/z:433.1604[m-h]-,calcd for c
24h24
o4f
3 433.1632.
[0366]
实施例105:(e)-4-(环丙基甲氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t3)的制备
[0367][0368]
参照化合物c1的合成,以化合物8t-3(150mg,0.33mmol)为原料得到化合物t3,黄色粉末79mg,收率为54%,纯度为95.32%,熔点为171.6-172.3℃;1h nmr(400mhz,cdcl3)δ11.49(s,1h),7.91(d,j=15.9hz,1h),7.60(q,j=8.4hz,4h),6.79(d,j=15.9hz,1h),6.59(s,1h),5.27(t,j=7.0hz,1h),3.97(d,j=7.0hz,2h),3.43(d,j=7.1hz,2h),1.83(s,3h),1.70(s,3h),1.38
–
1.24(m,1h),0.67(q,j=5.0hz,2h),0.40(q,j=5.0hz,2h).
13
c nmr(101mhz,cdcl3)δ176.1,162.8,162.1,141.3,140.9,133.1,132.0,129.4(q,2j
c-f
=32.3hz),129.3,127.0,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.0,117.7,104.6,103.0,73.2,26.0,22.4,18.0,10.4,3.4;esi-lrms m/z:444.9[m-h]-;esi-hrms m/z:445.1628[m-h]-,calcd for c
25h24
o4f
3 445.1632.
[0369]
实施例106:(e)-4-(环丁氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲
基)苯乙烯基)苯甲酸(t4)的制备
[0370][0371]
参照化合物t4的合成,以化合物8t-4(132mg,0.29mmol)为原料得到化合物t4,黄色粉末70mg,收率为55%,纯度为96.40%,熔点为157.4-158.1℃;1h nmr(400mhz,cdcl3)δ11.49(s,1h),7.90(d,j=15.9hz,1h),7.73
–
7.54(m,4h),6.76(d,j=15.9hz,1h),6.48(s,1h),5.25(t,j=7.1hz,1h),4.81(p,j=7.0hz,1h),3.39(d,j=7.1hz,2h),2.55
–
2.41(m,2h),2.33
–
2.14(m,2h),1.98
–
1.72(m,2h),1.82(s,3h),1.70(s,3h).
13
c nmr(101mhz,cdcl3)δ176.1,162.9,160.8,141.1,140.9,133.2,132.0,129.3,129.7(q,2j
c-f
=32.3hz),127.0,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=270.7hz),121.9,117.7,105.3,102.9,72.1,30.9,26.0,22.4,18.0,13.5;esi-lrms m/z:444.8[m-h]-;esi-hrms m/z:445.1627[m-h]-,calcd for c
25h24
o4f
3 445.1632.
[0372]
实施例107:(e)-4-(环戊氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t5)的制备
[0373][0374]
参照化合物c1的合成,以化合物8t-5(232mg,0.49mmol)为原料得到化合物t5,白色粉末178mg,收率为79%,纯度为95.99%,熔点为164.8-165.6℃;1h nmr(300mhz,cdcl3)δ11.53(s,1h),7.94(d,j=15.9hz,1h),7.71
–
7.57(m,4h),6.80(d,j=15.9hz,1h),6.66(s,1h),5.30
–
5.13(m,1h),5.05
–
4.91(m,1h),3.38(d,j=7.1hz,2h),2.05
–
1.65(m,14h).
13
c nmr(75mhz,cdcl3)δ176.0,162.9,161.2,141.0,141.0,133.3,131.8,129.2,129.2(q,2j
c-f
=32.3hz),127.0,125.8(q,3j
c-f
=3.8hz),124.4(q,2j
c-f
=270.2hz),122.0,118.0,105.7,102.6,80.0,33.2,26.0,24.2,22.4,18.0;esi-lrms m/z:458.8[m-h]-;esi-hrms m/z:459.1773[m-h]-,calcd for c
26h26
o4f
3 459.1789.
[0375]
实施例108:(e)-4-(环己氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t6)的制备
[0376][0377]
参照化合物c1的合成,以化合物8t-6(132mg,0.27mmol)为原料得到化合物t6,白色固体78mg,收率为61%,纯度为95.66%,熔点为168.9-169.7℃;1h nmr(400mhz,cdcl3)δ11.51(s,1h),7.92(d,j=15.9hz,1h),7.61(q,j=8.2hz,4h),6.77(d,j=15.9hz,1h),6.62(s,1h),5.25(t,j=7.1hz,1h),4.57
–
4.41(m,1h),3.40(d,j=7.1hz,2h),2.05
–
1.83
(m,4h),1.81(s,3h),1.69(s,3h),1.66
–
1.38(m,6h).
13
c nmr(101mhz,cdcl3)δ176.0,163.2,161.1,141.1,141.0,133.3,131.8,129.7(q,2j
c-f
=32.3hz),129.2,127.1,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=273.7hz),122.2,118.4,105.6,102.6,75.5,31.9,26.0,25.7,23.5,22.5,18.1;esi-lrms m/z:472.8[m-h]-;esi-hrms m/z:473.1936[m-h]-,calcd for c
27h30
o4f3473.1945.
[0378]
实施例109:(e)-4-(环庚氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t7)的制备
[0379][0380]
参照化合物c1的合成,以化合物8t-7(234mg,0.47mmol)为原料得到化合物t7,白色固体175mg,收率为77%,纯度为97.54%,熔点为160.5-161.3℃;1h nmr(400mhz,cdcl3)δ11.51(s,1h),7.93(d,j=15.9hz,1h),7.70
–
7.54(m,4h),6.78(d,j=15.9hz,1h),6.59(s,1h),5.25(t,j=6.9hz,1h),4.75
–
4.60(m,1h),3.40(d,j=7.0hz,2h),2.12
–
2.00(m,2h),1.86
–
1.94(m,2h),1.82(s,3h),1.80
–
1.74(m,2h),1.70(s,3h),1.67
–
1.61(m,4h),1.58
–
1.47(m,2h).
13
c nmr(101mhz,cdcl3)δ176.3,163.1,161.1,141.1,141.0,133.3,131.8,129.6(q,2j
c-f
=33.3hz),129.2,127.1,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=270.2hz),122.1,118.3,105.7,102.5,78.1,34.0,28.7,26.0,23.0,22.5,18.1;esi-lrms m/z:486.9[m-h]-;esi-hrms m/z:487.2084[m-h]-,calcd for c
28h30
o4f
3 487.2102.
[0381]
实施例110:(e)-4-(苄氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t8)的制备
[0382][0383]
参照化合物c1的合成,以化合物8t-8(227mg,0.46mmol)为原料得到化合物t8,白色粉末166mg,收率为75%,纯度为95.40%,熔点为196.9-197.5℃;1h nmr(400mhz,dmso-d6)δ8.00(d,j=16.1hz,1h),7.73(dd,j=23.1,8.3hz,4h),7.40(m,5h),7.04(d,j=16.1hz,1h),6.93(s,1h),5.28(s,2h),5.15(t,j=7.1hz,1h),3.31(d,j=7.1hz,2h),1.62(s,3h),1.61(s,3h).
13
c nmr(101mhz,dmso-d6)δ173.2,160.9,159.8,141.4,139.1,136.9,132.7,130.8,128.5,128.4,127.9,127.7(q,2j
c-f
=32.3hz),127.6,127.1,125.7(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=270.7hz),122.1,116.3,105.2,103.8,69.7,25.5,21.9,17.6;esi-lrms m/z:480.8[m-h]-;esi-hrms m/z:481.1614[m-h]-,calcd for c
28h24
o4f
3 481.1632.
[0384]
实施例111:(e)-4-(仲丁氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t9)的制备
[0385][0386]
参照化合物c1的合成,以化合物8t-9(92mg,0.19mmol)为原料得到化合物t9,黄色晶体68mg,收率为76%,纯度为95.32%,熔点为150.5-151.1℃;1h nmr(400mhz,cdcl3)δ11.51(s,1h),7.93(d,j=15.9hz,1h),7.70
–
7.55(m,4h),6.78(d,j=15.9hz,1h),6.62(s,1h),5.23(t,j=6.6hz,1h),4.62
–
4.47(m,1h),3.39(d,j=7.1hz,2h),1.89
–
1.61(m,2h),1.80(s,3h),1.69(s,3h),1.37(d,j=6.1hz,3h),1.02(t,j=7.4hz,3h).
13
c nmr(101mhz,cdcl3)δ176.0,163.1,161.4,141.1,141.0,133.3,131.8,129.7(q,2j
c-f
=31.3hz),128.5,126.8,125.9(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.1,118.3,105.4,102.6,75.3,29.4,25.9,22.5,19.6,18.0,9.8;esi-lrms m/z:446.9[m-h]-;esi-hrms m/z:447.1750[m-h]-,calcd for c
25h26
o4f
3 447.1789.
[0387]
实施例112:(e)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(戊-3-基氧基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t10)的制备
[0388][0389]
参照化合物c1的合成,以化合物8t-10(150mg,0.31mmol)为原料得到化合物t10,黄色晶体96mg,收率为67%,纯度为95.68%,熔点为161.5-162.1℃;1h nmr(400mhz,cdcl3)δ11.52(s,1h),7.93(d,j=15.9hz,1h),7.72
–
7.52(m,4h),6.78(d,j=15.9hz,1h),6.61(s,1h),5.23(t,j=7.1hz,1h),4.40(p,j=5.6hz,1h),3.40(d,j=7.1hz,2h),1.80(s,3h),1.76(m,4h),1.69(s,3h),0.98(d,j=7.4hz,6h).
13
c nmr(101mhz,cdcl3)δ176.0,163.1,161.8,141.1,141.0,133.4,131.7,130.0(q,2j
c-f
=32.3hz),129.2,127.1,125.9(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.2,118.1,105.3,102.5,80.1,26.3,25.9,22.5,18.0,9.6;esi-lrms m/z:460.9[m-h]-;esi-hrms m/z:461.1938[m-h]-,calcd for c
26h28
o4f3461.1945.
[0390]
实施例113:(e)-4-(己-3-基氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t11)的制备
[0391][0392]
参照化合物c1的合成,以化合物8t-11(132mg,0.27mmol)为原料得到化合物t11,白色粉末99mg,收率为77%,纯度为95.40%,熔点为126.7-127.5℃;1h nmr(400mhz,cdcl3)δ11.54(s,1h),7.92(d,j=15.9hz,1h),7.60(q,j=8.6hz,4h),6.77(d,j=15.9hz,1h),6.61(s,1h),5.21(t,j=7.1hz,1h),4.57
–
4.30(m,1h),3.39(d,j=7.1hz,2h),1.79(s,
3h),1.78
–
1.55(m,4h),1.67(d,j=7.6hz,3h),1.52
–
1.31(m,2h),0.97(q,j=7.5hz,6h).
13
c nmr(101mhz,cdcl3)δ175.9,163.1,161.8,141.1,141.0,133.4,131.7,129.6(q,2j
c-f
=33.3hz),129.1,127.0,125.8(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=272.7hz),122.1,118.1,105.2,102.5,78.7,35.78,26.8,25.9,22.5,18.7,18.0,14.3,9.5;esi-lrms m/z:474.8[m-h]-;esi-hrms m/z:475.2099[m-h]-,calcd for c
27h30
o4f
3 475.2102.
[0393]
实施例114:(e)-4-(2-氟乙氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t12)的制备
[0394][0395]
参照化合物c1的合成,以化合物8t-12(134mg,0.30mmmol)为原料得到化合物t12,黄色晶体113mg,收率为87%,纯度为96.18%,熔点为160.2-160.8℃;1h nmr(400mhz,cdcl3)δ11.50(s,1h),7.90(d,j=15.9hz,1h),7.62(q,j=8.7hz,4h),6.80(d,j=15.9hz,1h),6.61(s,1h),5.24(t,j=7.2hz,1h),5.00
–
4.62(m,2h),4.53
–
4.21(m,2h),3.42(d,j=7.1hz,2h),1.81(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ175.9,162.9,161.4,141.3,140.8,132.9,132.3,129.8(q,2j
c-f
=32.3hz),129.5,127.1,125.9(q,3j
c-f
=4.0hz),124.4(q,1j
c-f
=273.7hz),121.7,118.1,104.4,103.7,81.8(d,1j
c-f
=172.7hz),67.8(q,2j
c-f
=21.2hz),25.9,22.4,17.9;esi-lrms m/z:436.8[m-h]-;esi-hrms m/z:437.1382[m-h]-,calcd for c
23h21
o4f
4 437.1381.
[0396]
实施例115:(e)-4-(2,2-二氟乙氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t13)的制备
[0397][0398]
参照化合物c1的合成,以化合物8t-13(182mg,0.39mmmol)为原料得到化合物t13,黄色粉末108mg,收率为61%,纯度为96.65%,熔点为166.2-167.1℃;1h nmr(400mhz,cdcl3)δ11.50(s,1h),7.89(d,j=15.9hz,1h),7.73
–
7.53(m,4h),6.81(d,j=15.9hz,1h),6.58(s,1h),6.14(tt,j=55.0,4.0hz,1h),5.21(t,j=7.1hz,1h),4.33(td,j=12.8,4.0hz,2h),3.41(d,j=7.1hz,2h),1.79(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ175.8,163.0,160.4,141.5,140.7,132.5,132.5,129.9(q,2j
c-f
=33.3hz),129.9,127.1,125.9(q,3j
c-f
=4.0hz),125.7,124.4(q,1j
c-f
=272.7hz),121.4,118.3,113.5(t,1j
c-f
=243.4hz),104.2,104.2,67.6(t,2j
c-f
=30.3hz),25.9,22.4,17.9;esi-lrms m/z:454.8[m-h]-;esi-hrms m/z:455.1294[m-h]-,calcd for c
23h20
o4f
5 455.1287.
[0399]
实施例116:(e)-2-羟基-3-(3-甲基丁-2-烯-1-基)-4-(2,2,2-三氟乙氧基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t14)的制备
[0400][0401]
参照化合物c1的合成,以化合物8t-14(132mg,0.27mmol)为原料得到化合物t14,白色固体98mg,收率为76%,纯度为95.28%,熔点为156.1-156.9℃;1h nmr(400mhz,cdcl3)δ11.51(s,1h),7.89(d,j=15.9hz,1h),7.63(q,j=8.5hz,4h),6.80(d,j=15.9hz,1h),6.55(s,1h),5.22(t,j=7.0hz,1h),4.49(q,j=7.9hz,2h),3.43(d,j=7.0hz,1h),1.79(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ175.8,163.1,159.8,141.5,140.6,132.7,132.4,130.0,129.9(q,2j
c-f
=32.3hz),127.1,125.8(q,3j
c-f
=4.0hz),124.3(q,1j
c-f
=272.7hz),123.1(q,1j
c-f
=278.8hz),121.1,118.6,104.6,104.2,65.9(q,1j
c-f
=36.4hz),25.9,22.4,17.8;esi-lrms m/z:472.7[m-h]-;esi-hrms m/z:473.1182[m-h]-,calcd for c
23h19
o4f6473.1193.
[0402]
实施例117:(e)-4-(3-氟丙氧基)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)苯甲酸(t15)的制备
[0403][0404]
参照化合物c1的合成,以化合物8t-15(176mg,0.38mmol)为原料得到化合物t15,白色粉末131mg,收率为77%,纯度为95.15%,熔点为173.4-174.0℃;1h nmr(400mhz,cdcl3)δ11.50(s,1h),7.91(d,j=15.9hz,1h),7.66
–
7.58(q,j=8.5hz,4h),6.82(d,j=15.9hz,1h),6.66(s,1h),5.21(t,j=6.5hz,1h),4.69(dt,j=47.0,5.7hz,2h),4.27(t,j=6.0hz,2h),3.39(d,j=7.0hz,2h),2.25(dp,j=26.0,6.0hz,2h),1.79(s,3h),1.69(s,3h).
13
c nmr(101mhz,cdcl3)δ176.0,162.8,161.7,141.4,140.9,132.9,132.0,129.7(q,2j
c-f
=33.3hz),129.5,127.1,125.9(q,3j
c-f
=4.0hz),124.4(q,2j
c-f
=272.7hz),121.9,117.6,104.4,103.3,80.7(d,1j
c-f
=165.6hz),64.2(d,3j
c-f
=5.1hz),30.6(d,2j
c-f
=20.2hz),25.9,22.4,18.0;esi-lrms m/z:450.8[m-h]-;esi-hrms m/z:451.1502[m-h]-,calcd for c
24h22
o4f4451.1538.
[0405]
实施例118:(e)-2-羟基-3-(3-甲基丁-2-烯-1-基)-6-(4-(三氟甲基)苯乙烯基)-4-(3,3,3-三氟丙氧基)苯甲酸(t16)的制备
[0406][0407]
参照化合物c1的合成,以化合物8t-16(188mg,0.37mmol)为原料得到化合物t16,黄色晶体117mg,收率为65%,纯度为95.88%,熔点为181.3-182.1℃;1h nmr(400mhz,
lrms m/z:500.8[m-h]-;esi-hrms m/z:501.1538[m-h]-,calcd for c
25h23
o4f 501.1506.
[0414]
实施例121:最低抑菌浓度(mic)实验
[0415]
(1)实验方法:用mh肉汤培养基配制化合物浓度为128μg/ml,取96孔板,孔板b、c行加入化合物100μl,每个化合物三个复孔,b及至g行加入100μl的mh肉汤培养基,b行混匀后用排枪取100μl置于c行,混匀然后取100μl置于d行,依次类推至g行;g行混匀后取出100μl弃去。将细菌密度调节为5
×
10^5cfu/ml,每孔再加入100μl含细菌新鲜培养基。边缘孔用200μl无菌pbs填充以防培养基蒸发导致的边缘效应。37℃培养24小时后记录细菌生长情况,以无菌落生长的最小化合物浓度对应即为该化合物最小抑菌浓度(mic)。以氨苄西林(ampicillin)和诺氟沙星(norfloxacin)为阳性对照,实验结果见表1和表2。
[0416]
(2)试验结果:抗菌结果见表1及表2,木豆素衍生物对一株金黄色葡萄球菌和11株mrsa的mic范围在1~16μg/ml(y7除外),优于母核木豆素。
[0417]
表1实施例制备的木豆素衍生物的抑菌活性
[0418]
[0419][0420]
表2实施例制备的木豆素衍生物的抑菌活性
[0421]
[0422]
[0423][0424]
a staphylococcus aureus atcc25923.
[0425]
b mrsa atcc43300.
[0426]
e~f为4株临床mrsa分离株,由广州军区广州总医院赠送
[0427]
g~l为6株临床mrsa分离株,由深圳市人民医院馈赠
[0428]
m escherichia coli atcc25922.
[0429]
n pseudomonas aeruginosa atcc27853.
[0430]
o enterococcus faecalis atcc29212.
[0431]
实施例122:细胞毒性mtt实验
[0432]
(1)实验方法:
[0433]
1、种板:取生长对数期的小鼠巨噬细胞raw264.7、正常人肝细胞lo2,消化之后以5000个细胞每孔的细胞密度接种到96孔板,每孔100μl,边缘孔用200μl无菌pbs填充以防培养基蒸发导致的边缘效应,培养过夜待细胞贴壁。
[0434]
2、配药:将化合物(用细胞级dmso配置,母液浓度为10mm)用含血清dmem培养基稀释至10μm。
[0435]
3、加药:将细胞上清液轻轻吸走并吸干净,用上述已经配好的含药培养基和control组(加0.1%,v/v的dmso)代替原培养基,继续培养24小时,每个浓度设置5个复孔。
[0436]
4、加mtt:作用24小时后避光条件每孔加入20μl 5mg/ml配制好并过0.22μm微孔滤膜的mtt溶液,恒温培养箱继续培养4个小时。
[0437]
5、测板:将上清液轻轻吸干,不要触碰到底部细胞,加入150μl dmso振摇溶解甲瓒,用酶标仪测定570nm处波长的吸光值。
[0438]
细胞生长存活率(%)=100-(control
od值-化合物组
od值
)/control
od值
×
100%
[0439]
(2)试验结果:10μg/ml的化合物对两种哺乳动物细胞的mtt毒性实验结果如图1所示,其中(a)为小鼠巨噬细胞raw264.7,(b)为正常人的肝细胞lo2,从中选取10μm的化合物中毒性最小且mic活性较好的化合物y8进一步做机制探究。
[0440]
实施例123:持留菌实验
[0441]
(1)实验方法:初筛实验挑取单克隆的耐甲氧西林金黄色葡萄球菌(atcc 43300)接种至新鲜的mh肉汤培养基中培养12~14小时(37℃,200rpm)至平台期,加入10倍mic的环丙沙星(环丙沙星的mic用本实验室条件做出结果为0.5μg/ml)继续培养12小时(37℃,200rpm)。离心(3500rpm,5min,4℃),收集菌体,无菌pbs洗涤两次;加入无菌pbs重新悬浮细菌,调节od值使得菌落密度为10^7cfu/ml。取200μl到96孔板中,加入不同浓度化合物,10倍mic的达托霉素(达托霉素需加终浓度为50μg/ml的cacl2)作为阳性对照药,0.1%(v/v)的
dmso作为阴性对照药。加药(10μg/ml)完成后混合均匀,37℃培养箱培养,在12个小时每孔取出100μl点在mh琼脂平板上,培养18小时后取出拍照,结果见图2,图2中c1~t14为我们的化合物,每组均设置dmso做阴性对照,达托霉素(dapt.)作为抗生素对照,结果发现,10μg/ml的木豆素衍生物中,c2、c3、c4、c5、y1、y3、y4、y5、y6、y8、y9、t4、t5、t6、t7、t10、t11、t13、t14、t15、t16、t17、t18可以清除持留菌,37个化合物中有23个化合物作用持留菌12个小时可以清除持留菌,说明这类木豆素衍生物对mrsa持留菌有良好的效果。
[0442]
结合mic和mtt数据,根据持留菌初筛的结果,选择y8化合物作为活性化合物,做持留菌杀菌曲线实验。持留菌培养同初筛实验,加入不同浓度梯度的化合物(2倍mic、4倍mic、6倍mic)以及阳性对照10倍mic的达托霉素(达托霉素需加终浓度为50μg/ml的cacl2)、100倍mic的环丙沙星和100倍mic的利福平,0.1%(v/v)的dmso作为阴性对照。,混匀后置于培养箱培养。在2小时、4小时、6小时、8小时取样,系列稀释,每一稀释梯度取100μl用一次性涂布棒均匀涂布在mh琼脂平板上,37℃培养箱培养24小时后记录菌落数,并绘制曲线,结果见图3,从图3中可以看出,与dmso相比,4倍、6倍mic的y8可以在4小时内清除持留菌,2倍mic在8小时可以清除持留菌,效果明显优于10倍mic的达托霉素,100倍mic的环丙沙星和利福平。说明化合物y8具有很好的清除持留菌的活性。这充分证明了这类木豆素衍生物具备良好的清除持留菌的效果。
[0443]
实施例124:生物膜清除实验
[0444]
(1)实验方法:挑取单克隆的耐甲氧西林金黄色葡萄球菌(atcc 43300)接种至新鲜的mh肉汤培养基3ml培养过夜,次日取出按1:100用新鲜培养基稀释继续培养6小时(37℃,200rpm)至对数生长期,取出按1:100稀释。取稀释后的菌液200μl加至96孔板,周围加无菌mh肉汤培养基,放置37℃培养箱培养24小时。在无菌条件下,吸弃原菌液,用无菌pbs洗涤四次。加用新鲜mh肉汤培养基配制的不同浓度化合物(1倍mic、2倍mic、4倍mic、8倍mic)以及相同体积分数control组阴性对照dmso(0.1%v/v)和阳性对照组万古霉素(van.8倍mic,mic为本实验室条件下测定的值为0.5μg/ml),blank为只加培养基的空白对照组。继续放置37℃培养箱培养18小时。取出,吸弃菌液,pbs洗涤三次,风干。加0.1%结晶紫染色30分钟,吸弃结晶紫,pbs洗涤三次,风干。加30%醋酸200μl,震荡15分钟。用酶标仪570nm检测吸光值。生物膜抑制率=[(control
od值-blank
od值
)-(化合物组
od值-blank
od值
)]/(control
od值-blank
od值
)
×
100%。
[0445]
(2)试验结果:不同浓度化合物y8清除生物膜检测结果如图4所示,生物膜的含量随着化合物浓度增加而减少,阳性对照万古霉素没有明显变化,说明化合物y8具有良好的清除mrsa生物膜的活性且呈现浓度依赖性。
[0446]
实施例125:小鼠皮肤脓肿实验
[0447]
(1)实验方法:
[0448]
1、动物准备:昆明小鼠40只(购自省动物中心,雄性),买来后饲养观察10天。造模前一天,小鼠用脱毛膏背部脱毛,随机分组,每组8只。
[0449]
2、菌液准备:提前挑取单克隆的耐甲氧西林金黄色葡萄球菌(atcc 43300)接种至新鲜的mh肉汤培养基中培养12~14小时(37℃,200rpm)至至平台期,加入10倍mic的环丙沙星孵育12小时;离心(3500rpm,5min,4℃)收集菌体,无菌pbs洗涤两次;加入无菌pbs重新悬浮细菌,调节od值使得菌落密度为10^8cfu/ml,放置冰上备用。
[0450]
3、造模:用戊巴比妥钠(1%)按50mg/kg麻醉小鼠,待小鼠麻醉后,手提起小鼠背部皮肤,注射菌液100μl。放回笼内,30分钟后观察小鼠苏醒情况。
[0451]
4、给药:小鼠称重,细菌感染24小时后,根据小鼠体重皮下给药,分别给予化合物组,用生理盐水配,浓度分别200mg/kg(即每1kg的体重对应加入200mg的化合物)、100mg/kg、50mg/kg;阳性对照组,100mg/kg万古霉素(van.)、50mg/kg万古霉素(van.);阴性对照组(生理盐水,control),每隔24小时给药,连续给药4天。
[0452]
5、评价:给药4天后,水合氯醛麻醉小鼠,取皮肤脓肿组织,称量重量。取心、肝、脾、肺、肾和脓肿皮肤,生理盐水洗净,立即用组织固定液固定,放置4℃,送公司做组织切片,he染色。小鼠脓肿组织称量重量(ep管提前在超净工作台盖上盖子,称重,质量记录为m1,取出脓肿组织后再称重为m2,脓肿组织质量=m
2-m1,用组织匀浆机匀浆,匀浆三次左右,每次10秒直至组织完全破碎。取96孔板,每孔提前加180μl无菌pbs,取匀浆液20μl,加至第一列,每组三个复孔,倍半稀释法稀释101、102、103、104、105、106、107稀释梯度。每个稀释梯度取20μl,加至mh琼脂平板。放置37℃培养24小时菌落计数,结果如图5所示。取小鼠内脏组织做he染色,结果如图6所示。
[0453]
(2)试验结果:与生理盐水组control相比,化合物组可以降低mrsa持留细菌负荷量,其中200mg/kg浓度可以降低约1.2个log/cfu,具有明显的显著性差异(p﹤0.01),100mg kg-1
和50mg kg-1
的化合物y8可以将菌落数下降约1个cfu,具有显著性差异(p﹤0.05)。阳性对照万古霉素高浓度100mg kg-1可以显著降低菌落数(p﹤0.001),而较低浓度50mg kg-1
则无显著性差异。证明化合物y8在体内表现出良好的抗持留菌活性。此外,病理组织切片如图6所示,加药组与对照组没有显著差异,证明无明显毒性。
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。