一种
γ-巯基-β-磺酰基丁酸甲酯的合成方法
技术领域
1.该专利涉及有机合成、药物合成、有机化工的研究领域,具体的方法就是在催化剂量的布朗斯特酸催化下,(杂)芳基/烷基亚磺酸钠与2-甲氧基噻吩在水中室温下合成γ-巯基-β-苯磺酰基丁酸甲酯。
背景技术:
2.有机硫分子广泛存在于自然界和日常生活中(shen,c.,zhang,p.,sun,q.,bai,s.,hor,t.a.,liu,x.chem.soc.rev.,2015,44,291-314),经常出现在一些药物(haruki,h.,pedersen,m.g.,gorska,k.i.,pojer,f.,johnsson,k.science,2013,340,987-991)、天然产品(sobolewska,d.,podolak,i.,makowska-j.allium ursinum:botanical,phytochemical and pharmacological overview.phytochem.rev.,2015,14,81-97)和有机材料中(lang,f.,song,l.,lin,y.,you,y.,li,d.,jiang,q.j.appl.polym.sci.,2021,138,50750),含硫化合物在有机硫化学中起着重要的作用,它广泛存在于药物分子中,例如,偏头痛药物relpax(kumar,u.s.,sankar,v.r.,rao,m.m.,jaganathan,t.s.,buchi reddy,r.org.process res.dev.,2012,16,1917-1920)、青光眼治疗药物dorzolamide(ali,b.m.,zaitone,s.a.,shouman,s.a.,moustafa,y.m.naunyn-schmiedeberg's arch.pharmacol.,2015,388,1271-1282)、肾素抑制剂remikiren(doswald,s.,estermann,h.,kupfer,e.,stadler,h.,walther,w.,weisbrod,t.,wostl,w.bioorg.med.chem.,1994,2,403-410)和麻风病治疗药物dapsone(basak,a.,goswami,m.,rajkumar,a.,mitra,t.,majumdar,s.,o’reilly,p.,m.bdour,h.,l.trudeau,t.,basak,a.bioorg.med.chem.lett.,2015,25,2225-2237)。此外,硫醇作为一种含硫化合物,在制药、农业等行业也有多种应用。例如,正辛基硫醇作为聚合改性剂或硫化改性剂在橡胶合成中发挥了关键作用(cairns,t.l.,larchar,a.w.,mckusick,b.c.j.org.chem.,1953,18,748-752)。
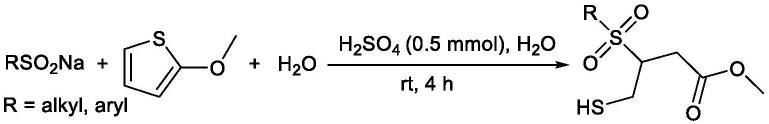
3.我们在此报告了一种在水中进行布朗斯特酸催化2-甲氧基噻吩的开环反应,在水中室温下合成γ-巯基-β-苯磺酰基丁酸甲酯。
4.尽我们所知,未见与本技术相同的文献报道。
技术实现要素:
5.本发明提供一种γ-巯基-β-苯磺酰基丁酸甲酯的合成方法。
6.本发明公开的γ-巯基-β-苯磺酰基丁酸甲酯的合成方法均一步完成,即在水中,布朗斯特酸催化(杂)芳基/烷基亚磺酸钠和2-甲氧基噻吩开环一步合成γ-巯基-β-苯磺酰基丁酸甲酯,反应通式如下。亚磺酸钠为芳基亚磺酸钠和烷基亚磺酸钠。
β-苯磺酰基(4-叔丁基)丁酸甲酯产率为63%。
24.产物γ-巯基-β-苯磺酰基(4-叔丁基)丁酸甲酯的结构表征数据如下:
[0025]1h nmr(400mhz,chloroform-d)δ(ppm):1.36(s,9h),1.68(dd,j=9.4,8.3,1h),2.73(m,1h),2.94(m,2h),3.14(ddd,j=14.4,8.3,4.3,1h),3.67(m,4h),7.59(m,2h),7.81(m,2h).
[0026]
13
c nmr(101mhz,chloroform-d)δ(ppm):22.90,31.08,31.56,35.38,52.34,62.98,126.51,128.90,133.86,158.41,170.70.
[0027]
hrms(esi)calcd for c
15h23
o4s2[m h]
331.1038,found 331.1031;hrms(esi)calcd for c
15h22
nao4s2[m na]
353.0857,found 353.0851;hrms(esi)calcd for c
15h22
ko4s2[m k]
369.0597,found 360.0603.
[0028]
实施例四
[0029]
4-甲氧基苯亚磺酸钠代替实施例一中的苯亚磺酸钠,得到黄色油状液体γ-巯基-β-苯磺酰基(4-甲氧基)丁酸甲酯产率为52%。
[0030]
产物γ-巯基-β-苯磺酰基(4-甲氧基)丁酸甲酯的结构表征数据如下:
[0031]1h nmr(400mhz,chloroform-d)δ(ppm):1.67(dd,j=9.4,8.2,1h),2.71(dt,j=14.3,9.1,1h),2.92(m,2h),3.13(ddd,j=14.2,8.3,4.3,1h),3.66(m,4h),3.90(s,3h),7.04(m,2h),7.81(m,2h).
[0032]
13
c nmr(101mhz,chloroform-d)δ(ppm):22.94,31.67,52.37,55.82,63.23,114.69,128.20,131.22,164.27,170.77.
[0033]
hrms(esi)calcd for c
12h16
nao5s2[m na]
327.0337,found 327.0325;hrms(esi)calcd for c
12h16
ko5s2[m k]
343.0076,found 343.0070.
[0034]
实施例五
[0035]
2-氟苯亚磺酸钠代替实施例一中的苯亚磺酸钠,得到黄色油状液体γ-巯基-β-苯磺酰基(2-氟)丁酸甲酯产率为39%。
[0036]
产物γ-巯基-β-苯磺酰基(2-氟)丁酸甲酯的结构表征数据如下:
[0037]1h nmr(400mhz,chloroform-d)δ(ppm):1.72(d,j=17.9,1h),2.86(ddd,j=14.4,9.2,8.1,1h),2.96(m,2h),3.18(ddd,j=14.4,8.7,4.5,1h),3.61(s,3h),3.97(dtd,j=8.2,6.3,4.5,1h),7.28(ddd,j=9.6,8.2,1.1,1h),7.37(td,j=7.7,1.1,1h),7.70(m,1h),7.91(ddd,j=7.8,6.9,1.8,1h).
[0038]
13
c nmr(101mhz,chloroform-d)δ(ppm):22.46,31.37,52.32,62.61,117.48,117.69,124.89,124.93,125.29,125.43,131.45,136.90,136.99,158.65,161.21,170.42.
[0039]
hrms(esi)calcd for c
11h13
fnao4s2[m na]
315.0137,found 315.0123;hrms(esi)calcd for c
11h13
fko4s2[m k]
330.9876,found 330.9846.
[0040]
实施例六
[0041]
4-氟苯亚磺酸钠代替实施例一中的苯亚磺酸钠,得到黄色油状液体γ-巯基-β-苯磺酰基(4-氟)丁酸甲酯产率为45%。
[0042]
产物γ-巯基-β-苯磺酰基(4-氟)丁酸甲酯的结构表征数据如下:
[0043]1h nmr(400mhz,chloroform-d)δ(ppm):1.68(dd,j=9.4,8.3,1h),2.74(ddd,j=14.3,9.4,8.6,1h),2.93(qd,j=17.1,6.3,2h),3.14(ddd,j=14.3,8.3,4.4,1h),3.68(m,
4h),7.28(m,2h),7.93(m,2h).
[0044]
13
c nmr(101mhz,chloroform-d)δ(ppm):22.78,31.57,52.45,63.24,116.75,116.98,131.91,132.01,133.13,133.16,164.96,167.52,170.55.
[0045]
hrms(esi)calcd for c
11h13
fnao4s2[m na]
315.0137,found 315.0125.
[0046]
实施例七
[0047]
3-氟苯亚磺酸钠代替实施例一中的苯亚磺酸钠,得到黄色油状液体γ-巯基-β-苯磺酰基(3-氟)丁酸甲酯产率为38%。
[0048]
产物γ-巯基-β-苯磺酰基(3-氟)丁酸甲酯的结构表征数据如下:
[0049]1h nmr(400mhz,chloroform-d)δ(ppm):(dd,j=9.4,8.3,1h),2.68(ddd,j=14.3,9.3,8.5,1h),2.85(m,2h),3.06(ddd,j=14.4,8.4,4.4,1h),3.58(s,3h),3.67(dtd,j=8.5,6.3,4.4,1h),7.33(tdd,j=8.2,2.6,1.0,1h),7.54(m,2h),7.64(ddd,j=7.8,1.7,1.1,1h).
[0050]
13
c nmr(101mhz,chloroform-d)δ(ppm):22.72,31.45,52.39,63.12,116.20,116.44,121.58,121.79,124.82,124.86,131.34,131.42,139.18,139.25,161.25,163.77,170.44.
[0051]
hrms(esi)calcd for c
11h13
fnao4s2[m na]
315.0137,found 315.0143.
[0052]
实施例八
[0053]
4-溴苯亚磺酸钠代替实施例一中的苯亚磺酸钠,得到黄色油状液体γ-巯基-β-苯磺酰基(4-溴)丁酸甲酯产率为43%。
[0054]
产物γ-巯基-β-苯磺酰基(4-溴)丁酸甲酯的结构表征数据如下:
[0055]1h nmr(400mhz,chloroform-d)δ(ppm):(t,j=8.8,1h),2.66(dt,j=14.3,9.0,1h),2.80(dd,j=17.1,6.6,1h),2.89(dd,j=17.1,6.0,1h),3.05(ddd,j=14.3,8.2,4.4,1h),3.57(s,3h),3.64(dtd,j=8.7,6.3,4.4,1h),7.68(m,4h).
[0056]
13
c nmr(101mhz,chloroform-d)δ(ppm):22.73,31.51,52.41,63.17,129.86,130.52,132.80,136.12,170.47.
[0057]
hrms(esi)calcd for c
11h13
brnao4s2[m na]
374.9336,found 374.9326.
[0058]
实施例九
[0059]
4-氯苯亚磺酸钠代替实施例一中的苯亚磺酸钠,得到黄色油状液体γ-巯基-β-苯磺酰基(4-氯)丁酸甲酯产率为66%。
[0060]
产物γ-巯基-β-苯磺酰基(4-氯)丁酸甲酯的结构表征数据如下:
[0061]1h nmr(400mhz,chloroform-d)δ(ppm):1.70(dd,j=9.4,8.3,1h),2.73(dt,j=14.3,9.1,1h),2.88(dd,j=17.1,6.6,1h),2.97(dd,j=17.1,6.0,1h),3.13(ddd,j=14.3,8.3,4.4,1h),3.67(m,4h),7.58(m,2h),7.84(m,2h).
[0062]
13
c nmr(101mhz,chloroform-d)δ(ppm):22.74,31.52,52.39,63.19,129.82,130.50,135.55,141.22,170.50.
[0063]
hrms(esi)calcd for c
11h13
clnao4s2[m na]
330.9841,found 330.9826.hrms(esi)calcd for c
11h13
clko4s2[m k]
346.9581,found 346.9574.
[0064]
实施例十
[0065]
甲基亚磺酸钠代替实施例一中的苯亚磺酸钠,得到黄色油状液体γ-巯基-β-甲磺
酰基丁酸甲酯产率为78%。
[0066]
产物γ-巯基-β-甲磺酰基丁酸甲酯的结构表征数据如下:
[0067]1h nmr(400mhz,chloroform-d)δ(ppm):1.81(dd,j=9.3,8.5,1h),3.00(m,6h),3.19(ddd,j=14.4,8.5,5.0,1h),3.64(dtd,j=7.8,6.2,4.9,1h),3.76(s,3h).
[0068]
13
c nmr(101mhz,chloroform-d)δ(ppm):22.95,31.73,40.60,52.53,62.15,170.87.
[0069]
hrms(esi)calcd for c6h
12
nao4s2[m na]
235.0075,found 235.0065.
[0070]
实施例十一
[0071]
乙基苯亚磺酸钠代替实施例一中的苯亚磺酸钠,得到黄色油状液体γ-巯基-β-乙磺酰基丁酸甲酯产率为51%。
[0072]
产物γ-巯基-β-乙磺酰基丁酸甲酯的结构表征数据如下:
[0073]1h nmr(400mhz,chloroform-d)δ(ppm):1.35(t,j=7.4,3h),1.73(d,j=17.7,1h),2.83(m,2h),3.02(m,4h),3.58(dtd,j=7.7,6.3,5.1,1h),3.68(s,3h).
[0074]
13
c nmr(101mhz,chloroform-d)δ(ppm):5.09,22.03,30.71,46.26,51.48,170.01.
[0075]
hrms(esi)calcd for c7h
14
nao4s2[m na]
249.0231,found 249.0219.
[0076]
实施例十二
[0077]
2-噻吩亚磺酸钠代替实施例一中的苯亚磺酸钠,得到黄色油状液体γ-巯基-β-(2-噻吩)磺酰基丁酸甲酯产率为53%。
[0078]
产物γ-巯基-β-(2-噻吩)磺酰基丁酸甲酯的结构表征数据如下:
[0079]1h nmr(400mhz,chloroform-d)δ(ppm):1.72(dd,j=9.5,8.3,1h),2.77(ddd,j=14.3,9.4,8.5,1h),2.99(m,2h),3.21(ddd,j=14.3,8.4,4.4,1h),3.68(s,3h),3.80(dtd,j=8.5,6.3,4.4,1h),7.21(dd,j=5.0,3.8,1h),7.72(dd,j=3.8,1.4,1h),7.82(dd,j=5.0,1.4,1h).
[0080]
13
c nmr(101mhz,chloroform-d)δ(ppm):23.10,31.93,52.42,64.31,128.34,135.49,135.73,137.44,170.48.
[0081]
hrms(esi)calcd for c9h
12
nao4s3[m na]
302.9795,found 320.9794.hrms(esi)calcd for c9h
12
ko4s3[m k]
318.9539,found 318.9530.
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。