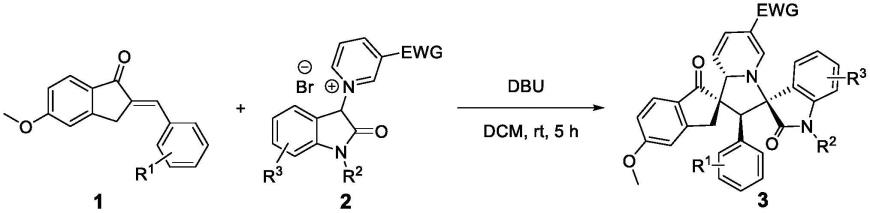
1.该专利涉及有机合成、药物合成、新药研发的研究领域,具体的方法是在dbu的促进下,茚酮衍生的α,β-不饱和酮和吲哚酮衍生的吡啶溴鎓盐发生1,3-偶极环加成反应生成一系列双螺环吡咯螺氧化吲哚类化合物;经过对立枯丝核、油菜菌核、番茄灰霉、小麦赤霉、辣椒疫霉等真菌的筛选,活性筛选结果显示:这些化合物有一定具有抗真菌的活性。
背景技术:
2.螺环化合物是非常有价值的结构单元和重要的药效团,广泛存在于各种天然生物碱和药物中。特别是含吡咯螺氧化吲哚骨架的螺环化合物,在药物化学上表现出较高的生物活性,例如从mitragyna speciosa树叶中分离的具有抗肿瘤活性的生物碱mitraphylline(n.bacher,m.tiefenthaler,s.sturm,h.stuppner,m.j.ausserlechner,r.kofler and g.konwalinka,brit.j.haematol.,2006,132,615-622.)、从植物horsfieldia superba中提取的具有止痛作用的吲哚螺环生物碱horsfiline(h.ripperger,a.preiss and m.d
í
az,phytochemistry,1983,22,2603-2605.)以及从aspergillus fumigatus的发酵液中分离出的具有癌细胞抑制作用的spirotryprostatin a和spirotryprostatin b生物碱(c.-b.cui,h.kakeya and h.j.osada,tetrahedron,1996,52,12651.),均具有典型的吡咯螺氧化吲哚结构。而近几年来,含吡咯螺氧化吲哚结构的双螺环化合物也陆续被报道在抗菌(k.karthikeyan,p.m.sivakumar,m.doble and p.t.perumal,eur.j.med.chem.,2010,45,3446-3452.)、抗结核(s.v.karthikeyan,b.d.bala,v.p.a.raja,perumal,s.p.yogeeswari and d.sriram,bioorg.med.chem.lett.,2010,20,350-353.)、抗糖尿病(r.murugan,s.anbazhagan and s.sriman narayanan,eur.j.med.chem.,2009,44,3272-3279.)和抗霉菌(r.ranjith kumar,s.perumal,p.senthilkumar,p.yogeeswari and d.sriram,eur.j.med.chem.,2009,44,3821-3829.)等方面有潜在的生物活性,吸引了研究人员的广泛关注,合成具有吡咯螺氧化吲哚结构的双螺环化合物已经逐渐成为热门。基于此类化合物的重要性,拓展新的含吡咯螺氧化吲哚骨架的双螺环化合物的合成方法不仅具有重要的学术价值,也具有潜在的应用价值和社会价值。
3.目前合成双螺环吡咯螺氧化吲哚化合物主要有以下两种方法:(1)通过a-氨基酸与吲哚酮经过原位脱羧缩合合成的甲亚胺叶立德与具有环外双键的偶极子发生[3 2]环加成反应(k.dutta,m.kumar,s.k.ghosh,a.das and r.chowdhury,synthetic.commun.,2019,49,444-455.)。(2)通过醛和氨基酸脱羧过程提供的甲亚胺叶立德与3-乙烯基取代的吲哚酮发生[3 2]环加成反应(l.wang,x.m.shi,w.p.dong,l.p.zhu and r.wang,chem.commun.,2013,49,3458-3460.)。上述两类合成方法主要得到具有相邻季碳中心的双螺环吡咯氧化吲哚化合物,并且存在反应时间长、反应步骤繁琐以及产物结构单一等缺点,因此开发高效、便捷并且结构新颖的双螺环吡咯氧化吲哚类的新方法具有极大的意义。
[0004]
我们在此报告了一种吲哚酮衍生的吡啶溴鎓盐和茚酮衍生的α,β-不饱和酮之间
的1,3-偶极环加成反应,开发了一种新的双螺环吡咯螺氧化吲哚类化合物的合成方法。与先前关于合成双螺环吡咯螺氧化吲哚化合物的报道不同,该新方案提供了具有两个处于间位的季碳中心的新型双螺环吡咯螺氧化吲哚产物,并且,在抗真菌活性筛选中显示这类化合物具有一定的抗真菌活性。
[0005]
尽我们所知,未见与本技术相同的文献报道。
技术实现要素:
[0006]
本发明公开双螺环吡咯螺氧化吲哚类化合物及其合成方法和抗真菌活性。
[0007]
本发明公开的双螺环吡咯螺氧化吲哚类化合物合成方法均一步完成,即在二氯甲烷的溶液条件下,以dbu为碱,吲哚酮衍生的吡啶溴鎓盐和茚酮衍生的α,β-不饱和酮发生1,3-偶极环加成反应一步合成双螺环吡咯螺氧化吲哚类化合物,反应方程式如下图所示。
[0008][0009]
部分双螺环吡咯螺氧化吲哚化合物的抗真菌活性筛选的结果如表1所示。
[0010]
表1.抗真菌活性筛选的结果
[0011]
[0012][0013]
结合下面的实施例,更详细地阐述本发明,但并不认为它们是对本发明范围的限制。
具体实施方式
[0014]
实施例一
[0015]
向装有搅拌子的25ml玻璃试管中加入2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮(0.5mmol)、1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐(1.0mmol)、dbu(1.6mmol)和5ml ch2cl2。将所得混合物在室温下搅拌5小时,反应期间通过tlc监测反应进程。反应完成后,在旋转蒸发仪上蒸发溶剂,残余物通过碱性氧化铝柱色谱纯化(石油醚:乙酸乙酯=3:1
–
1:1用作洗脱剂),纯化后以76%产率和15:1dr值得到黄色固体产物,其熔点为141℃。
[0016]
产物3aa的结构表征数据如下:
[0017]1h nmr(400mhz,cdcl3)δ7.75(dd,j=7.5,1.3hz,1h),7.69(d,j=8.5hz,1h),7.33(td,j=7.7,1.3hz,1h),7.19(td,j=7.6,1.0hz,1h),7.10(q,j=3.0hz,3h),7.03-6.96(m,3h),6.88(dd,j=8.5,2.3hz,1h),6.79(d,j=2.2hz,1h),6.72(d,j=7.8hz,1h),6.29(dt,j=10.2,1.8hz,1h),5.88(t,j=2.2hz,1h),4.57(dd,j=10.3,2.0hz,1h),4.35(s,1h),3.84(s,3h),3.58(s,3h),3.34(s,2h),3.02(s,3h).
[0018]
13
c nmr(101mhz,cdcl3)δ205.59,175.61,166.63,165.64,155.53,144.20,140.32,140.24,133.86,130.65,130.48,128.47,128.16,127.66,125.60,125.52,124.35,
123.88,122.68,115.70,115.63,110.33,109.40,109.32,108.70,108.63,98.88,74.74,70.68,70.64,62.95,59.86,59.79,55.65,55.54,50.68,50.60,38.43,25.92,25.87.
[0019]
hrms m/z(esi)calcd for c
33h28
n2o5[m na]
555.1890;found 555.1881.
[0020]
实施例二
[0021]
2,3-二氢-5-甲氧基-2-[(3-甲基苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以33%产率和30:1dr值得到黄色固体产物,其熔点为156℃。
[0022]
产物3ba的结构表征数据如下:
[0023]1h nmr(400mhz,cdcl3)δ7.69(dd,j=7.8,5.8hz,2h),7.31(td,j=7.7,1.3hz,1h),7.17(td,j=7.6,1.0hz,1h),7.02-6.95(m,2h),6.92-6.82(m,3h),6.81-6.75(m,2h),6.72(d,j=7.8hz,1h),6.29(dt,j=10.1,1.8hz,1h),5.84(t,j=2.1hz,1h),4.56(dd,j=10.2,2.1hz,1h),4.32(s,1h),3.83(s,3h),3.57(s,3h),3.33(s,2h),3.05(s,3h),2.14(s,3h).
[0024]
13
c nmr(101mhz,cdcl3)δ205.52,175.69,166.65,165.62,155.68,144.22,140.22,140.17,137.77,133.64,131.28,131.22,130.62,128.51,128.38,127.99,127.22,125.57,125.42,124.28,123.82,122.54,115.62,115.58,110.42,110.39,109.39,109.33,108.64,98.93,74.56,70.58,70.54,62.99,59.75,59.68,55.62,55.54,50.67,50.60,38.65,25.90,25.87,21.27,21.23.
[0025]
hrms m/z(esi)calcd for c
34h30
n2o5[m na]
569.2047;found 569.2050.
[0026]
实施例三
[0027]
2,3-二氢-5-甲氧基-2-[(4-甲基苯基)亚甲基]-1h-茚酮代替代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以56%产率和14:1dr值得到黄色固体产物,其熔点为156℃。
[0028]
产物3ca的结构表征数据如下:
[0029]1h nmr(400mhz,cdcl3)δ7.73(dd,j=7.5,1.2hz,1h),7.68(d,j=8.5hz,1h),7.32(td,j=7.8,1.3hz,1h),7.18(td,j=7.6,1.0hz,1h),6.97(t,j=1.1hz,1h),6.89(s,5h),6.79(d,j=2.2hz,1h),6.72(d,j=7.8hz,1h),6.28(ddd,j=10.2,2.2,1.4hz,1h),5.85(t,j=2.1hz,1h),4.56(ddd,j=10.1,2.1,0.8hz,1h),4.32(s,1h),3.84(s,3h),3.58(s,3h),3.33(s,2h),3.04(s,3h),2.18(s,3h).
[0030]
13
c nmr(101mhz,cdcl3)δ205.63,175.70,166.65,165.61,155.63,144.24,140.32,140.23,137.32,130.61,130.32,128.90,128.52,125.57,125.48,124.44,123.84,122.63,115.63,115.56,110.35,110.31,109.41,109.33,108.70,108.62,98.89,74.64,70.61,70.56,62.98,59.52,59.44,55.64,55.53,50.67,50.59,38.54,25.95,25.90,20.91,20.85.
[0031]
hrms m/z(esi)calcd for c
34h30
n2o5[m na]
569.2047;found 569.2048.
[0032]
实施例四
[0033]
2,3-二氢-5-甲氧基-2-[(3,4-二甲基苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以46%产率和7:1dr值得到黄色固体产物,其熔点为159℃。
[0034]
产物3da的结构表征数据如下:
[0035]1h nmr(400mhz,cdcl3)δ7.72-7.64(m,2h),7.30(td,j=7.7,1.4hz,1h),7.16(t,j=7.5hz,1h),6.96(s,1h),6.89-6.82(m,2h),6.77(dd,j=7.4,2.0hz,2h),6.75-6.69(m,2h),6.28(dt,j=10.3,1.8hz,1h),5.82(t,j=2.1hz,1h),4.55(dd,j=10.2,2.0hz,1h),4.31(s,1h),3.83(s,3h),3.57(s,3h),3.33(s,2h),3.07(s,3h),2.06(s,3h),2.03(s,3h).
[0036]
13
c nmr(101mhz,cdcl3)δ205.58,175.80,166.69,165.59,155.82,144.28,140.22,140.17,136.34,135.94,131.73,130.91,130.57,129.41,128.58,127.51,125.56,125.40,124.36,123.81,122.50,115.52,110.48,110.44,109.41,109.36,108.66,98.97,74.45,70.51,70.48,63.05,59.42,59.36,55.62,55.54,50.67,50.61,38.83,25.91,19.63,19.60,19.20,19.17.
[0037]
hrms m/z(esi)calcd for c
35h32
n2o5[m na]
583.2203;found 583.2206.
[0038]
实施例五
[0039]
2,3-二氢-5-甲氧基-2-[(4-叔丁基苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以60%产率和》99:1dr值得到黄色固体产物,其熔点为146℃。
[0040]
产物3ea的结构表征数据如下:
[0041]1h nmr(400mhz,cdcl3)δ7.77(dd,j=7.5,1.3hz,1h),7.69(d,j=8.5hz,1h),7.34(td,j=7.7,1.3hz,1h),7.19(td,j=7.6,1.0hz,1h),7.12-7.06(m,2h),6.97(t,j=1.1hz,1h),6.89(dd,j=9.5,2.6hz,3h),6.80(d,j=2.2hz,1h),6.73(d,j=7.8hz,1h),6.30-6.24(m,1h),5.86(t,j=2.2hz,1h),4.56(ddd,j=10.2,2.1,0.8hz,1h),4.33(s,1h),3.84(s,3h),3.57(s,3h),3.33(s,2h),3.00(s,3h),1.18(s,9h).
[0042]
13
c nmr(101mhz,cdcl3)δ205.83,175.64,166.66,165.59,155.65,150.40,144.29,140.47,140.31,130.74,130.54,130.12,129.96,128.57,125.60,125.09,124.97,124.62,123.80,122.75,122.64,115.67,115.51,110.26,109.50,109.33,108.71,108.56,98.78,74.74,70.68,70.61,63.04,59.29,59.17,55.71,55.51,50.71,50.58,38.40,34.32,31.17,31.03,25.92,25.82.
[0043]
hrms m/z(esi)calcd for c
37h36
n2o5[m na]
611.2516;found 611.2517.
[0044]
实施例六
[0045]
2,3-二氢-5-甲氧基-2-[(4-甲氧基苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以24%产率和4:1dr值得到黄色固体产物,其熔点为135℃。
[0046]
产物3fa的结构表征数据如下:
[0047]1h nmr(400mhz,cdcl3)δ7.73(d,j=7.4hz,1h),7.68(d,j=8.5hz,1h),7.35-7.29(m,1h),7.21-7.15(m,1h),6.98(s,1h),6.96-6.91(m,2h),6.91-6.84(m,1h),6.83-6.78(m,1h),6.72(d,j=7.8hz,1h),6.63(dd,j=9.1,2.4hz,2h),6.28(dt,j=10.3,1.8hz,1h),5.86(t,j=2.2hz,1h),4.55(dd,j=10.2,2.0hz,1h),4.30(s,1h),3.84(s,3h),3.66(s,3h),3.57(s,3h),3.33(d,j=2.7hz,2h),3.03(s,3h).
[0048]
13
c nmr(101mhz,cdcl3)δ205.70,175.70,166.61,165.60,158.87,155.56,144.20,140.35,140.28,131.68,130.56,128.46,125.52,125.43,124.49,123.81,122.60,
115.63,115.58,113.51,110.29,110.25,109.36,109.31,108.67,108.61,98.80,74.72,70.57,70.53,62.94,59.26,59.19,55.62,55.52,55.00,54.92,50.63,50.56,38.41,25.93,25.88.
[0049]
hrms m/z(esi)calcd for c
34h30
n2o6[m na]
585.1996;found 585.2000.
[0050]
实施例七
[0051]
2,3-二氢-5-甲氧基-2-[(3-氯苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以56%产率和5:1dr值得到黄色固体产物,其熔点为151℃。
[0052]
产物3ga的结构表征数据如下:
[0053]1h nmr(400mhz,cdcl3)δ7.75-7.67(m,2h),7.35(td,j=7.7,1.2hz,2h),7.22-7.18(m,2h),7.10(ddd,j=8.0,2.0,1.2hz,1h),7.07-7.01(m,2h),6.97(t,j=1.1hz,1h),6.94-6.87(m,2h),6.82(d,j=2.2hz,1h),6.76(d,j=7.8hz,1h),6.29(ddd,j=10.2,2.2,1.4hz,1h),5.85(t,j=2.1hz,1h),4.56(ddd,j=10.1,2.1,0.9hz,1h),4.31(s,1h),3.85(s,3h),3.58(s,3h),3.39-3.23(m,2h),3.07(s,3h).
[0054]
13
c nmr(101mhz,cdcl3)δ205.03,175.36,166.56,165.75,155.32,144.10,140.08,139.95,136.27,133.19,133.09,130.89,129.73,129.66,129.14,128.29,125.67,125.43,123.98,123.85,122.68,122.60,122.18,115.88,115.75,110.35,110.31,109.45,109.31,108.92,108.79,99.14,74.51,70.63,70.56,62.87,59.23,59.11,55.70,55.54,50.72,50.61,38.41,25.96,25.90.
[0055]
hrms m/z(esi)calcd for c
33h27
cln2o5[m na]
589.1501;found 589.1497.
[0056]
实施例八
[0057]
2,3-二氢-5-甲氧基-2-[(4-氯苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以71%产率和9:1dr值得到黄色固体产物,其熔点为152℃。
[0058]
产物3ha的结构表征数据如下:
[0059]1h nmr(400mhz,cdcl3)δ7.74(d,j=7.4hz,1h),7.69(d,j=8.5hz,1h),7.35(t,j=7.7hz,1h),7.20(t,j=7.6hz,1h),7.08(d,j=8.1hz,2h),6.96(d,j=7.4hz,3h),6.90(dd,j=8.4,2.3hz,1h),6.81(d,j=2.2hz,1h),6.75(d,j=7.8hz,1h),6.29(d,j=10.2hz,1h),5.87(t,j=2.2hz,1h),4.56(dd,j=10.1,2.1hz,1h),4.32(s,1h),3.85(s,3h),3.58(s,3h),3.31(q,j=17.6hz,2h),3.05(s,3h).
[0060]
13
c nmr(101mhz,cdcl3)δ205.28,175.42,166.57,165.75,155.30,144.14,140.17,140.08,133.75,132.43,131.89,130.83,128.40,128.32,125.66,125.49,124.09,123.98,122.70,115.85,115.78,110.29,110.26,109.43,109.34,108.88,108.80,99.07,74.64,70.65,70.61,62.86,59.09,59.01,55.69,55.57,50.72,50.63,38.32,26.01,25.96.
[0061]
hrms m/z(esi)calcd for c
33h27
cln2o5[m na]
589.1501;found 589.1505.
[0062]
实施例九
[0063]
2,3-二氢-5-甲氧基-2-[(3-溴苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以72%产率和6:1dr值得到黄色
固体产物,其熔点为145℃。
[0064]
产物3ia的结构表征数据如下:
[0065]1h nmr(400mhz,cdcl3)δ7.63(dd,j=8.0,5.4hz,2h),7.29(d,j=7.5hz,1h),7.21-7.16(m,1h),7.12(q,j=6.7,5.8hz,2h),6.91(d,j=6.0hz,3h),6.83(dd,j=8.6,2.2hz,1h),6.75(d,j=2.2hz,1h),6.69(d,j=7.8hz,1h),6.22(dt,j=10.2,1.7hz,1h),5.78(t,j=2.2hz,1h),4.49(dd,j=10.1,2.0hz,1h),4.22(s,1h),3.78(s,3h),3.51(s,3h),3.33-3.15(m,2h),3.00(s,3h).
[0066]
13
c nmr(101mhz,cdcl3)δ205.03,175.36,166.56,165.75,161.81,155.32,144.10,140.08,139.95,136.27,133.19,133.09,130.89,129.73,129.66,128.29,125.67,125.43,123.98,123.85,122.68,122.60,122.18,115.88,115.75,110.35,110.31,109.45,109.31,108.92,108.79,99.14,74.51,70.63,70.56,62.87,59.23,59.11,55.70,55.54,50.72,50.61,38.41,25.96,25.90.
[0067]
hrms m/z(esi)calcd for c
33h27
brn2o5[m na]
633.0996;found 633.0997.
[0068]
实施例十
[0069]
2,3-二氢-5-甲氧基-2-[(4-溴苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以74%产率和10:1dr值得到黄色固体产物,其熔点为155℃。
[0070]
产物3ja的结构表征数据如下:
[0071]1h nmr(400mhz,cdcl3)δ7.73(dd,j=7.5,1.3hz,1h),7.68(d,j=8.5hz,1h),7.34(td,j=7.8,1.3hz,1h),7.25-7.15(m,3h),6.96(s,1h),6.89(dd,j=9.3,2.6hz,3h),6.80(d,j=2.2hz,1h),6.75(d,j=7.8hz,1h),6.28(dt,j=10.2,1.8hz,1h),5.85(d,j=2.2hz,1h),4.56(dd,j=10.3,2.1hz,1h),4.30(s,1h),3.85(s,3h),3.57(s,3h),3.29(q,j=17.6hz,2h),3.05(s,3h).
[0072]
13
c nmr(101mhz,cdcl3)δ205.24,175.38,166.56,165.74,155.28,144.12,140.13,140.05,132.94,132.22,132.15,132.05,131.78,131.33,130.84,128.29,125.65,125.47,124.04,122.68,121.98,115.85,115.78,110.28,110.25,109.42,109.33,108.90,108.82,99.07,74.55,70.64,70.58,62.82,59.11,59.02,55.68,55.56,50.71,50.63,38.32,26.02,25.96.
[0073]
hrms m/z(esi)calcd for c
33h27
brn2o5[m na]
633.0996;found 633.0996.
[0074]
实施例十一
[0075]
2-[(4-氟苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以77%产率和23:1dr值得到黄色固体产物,其熔点为143℃。
[0076]
产物3ka的结构表征数据如下:
[0077]1h nmr(400mhz,cdcl3)δ7.75(dd,j=7.5,1.2hz,1h),7.69(d,j=8.5hz,1h),7.34(td,j=7.8,1.3hz,1h),7.20(td,j=7.6,1.0hz,1h),7.02-6.95(m,3h),6.89(dd,j=8.5,2.3hz,1h),6.80(ddd,j=8.7,6.0,2.5hz,3h),6.73(d,j=7.8hz,1h),6.28(ddd,j=10.2,2.2,1.4hz,1h),5.87(t,j=2.1hz,1h),4.56(ddd,j=10.2,2.1,0.8hz,1h),4.33(s,1h),3.85(s,3h),3.58(s,3h),3.39
–
3.24(m,2h),3.03(s,3h).
[0078]
13
c nmr(101mhz,cdcl3)δ205.44,175.53,166.60,165.73,155.32,144.15,140.25,132.31,132.24,130.77,129.64,129.61,128.39,125.66,125.52,124.27,123.99,122.75,115.78,115.27,115.05,114.31,110.26,109.41,109.37,108.76,99.02,74.78,70.68,62.86,59.06,59.01,55.66,55.60,50.70,50.65,38.31,25.96,25.93.
[0079]
hrms m/z(esi)calcd for c
33h27
fn2o5[m na]
573.1796;found 573.1796.
[0080]
实施例十二
[0081]
2,3-二氢-5-甲氧基-2-[(4-三氟甲基苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以71%产率和10:1dr值得到黄色固体产物,其熔点为158℃。
[0082]
产物3la的结构表征数据如下:
[0083]1h nmr(400mhz,cdcl3)δ7.77(dd,j=7.6,1.3hz,1h),7.70(d,j=8.5hz,1h),7.36(td,j=7.8,1.3hz,3h),7.21(td,j=7.6,1.0hz,1h),7.15(d,j=8.1hz,2h),6.96(t,j=1.1hz,1h),6.90(dd,j=8.5,2.3hz,1h),6.81(d,j=2.2hz,1h),6.76(d,j=7.8hz,1h),6.29(ddd,j=10.3,2.2,1.4hz,1h),5.88(t,j=2.1hz,1h),4.57(ddd,j=10.3,2.1,0.9hz,1h),4.41(s,1h),3.85(s,3h),3.58(s,3h),3.40-3.16(m,2h),3.04(s,3h).
[0084]
13
c nmr(101mhz,cdcl3)δ205.09,175.26,166.54,165.81,155.16,144.11,140.05,140.01,138.30,130.97,130.90,130.22,130.00,129.68,129.35,128.26,125.72,125.55,125.13,125.09,124.10,123.91,122.77,115.90,110.29,110.26,109.43,109.39,108.93,99.20,74.62,70.71,70.68,62.93,59.18,59.14,55.67,55.61,50.72,50.68,38.28,26.00,25.98.
[0085]
hrms m/z(esi)calcd for c
34h27
f3n2o5[m na]
623.1764;found 623.1772.
[0086]
实施例十三
[0087]
2,3-二氢-5-甲氧基-2-[(4-三氟甲氧基苯基)亚甲基]-1h-茚酮代替实施例一中的2,3-二氢-5-甲氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以53%产率和75:1dr值得到黄色固体产物,其熔点为133℃。
[0088]
产物3ma的结构表征数据如下:
[0089]1h nmr(400mhz,cdcl3)δ7.78(dd,j=7.5,1.2hz,1h),7.70(d,j=8.5hz,1h),7.36(td,j=7.7,1.3hz,1h),7.21(td,j=7.6,1.0hz,1h),7.07-7.01(m,2h),6.98-6.93(m,3h),6.91(dd,j=8.5,2.2hz,1h),6.82(d,j=2.2hz,1h),6.75(d,j=7.8hz,1h),6.28(ddd,j=10.3,2.2,1.5hz,1h),5.88(t,j=2.1hz,1h),4.56(ddd,j=10.2,2.1,0.9hz,1h),4.35(s,1h),3.86(s,3h),3.58(s,3h),3.30(q,j=17.5hz,2h),3.01(s,3h).
[0090]
13
c nmr(101mhz,cdcl3)δ205.34,175.35,166.56,165.76,155.19,148.66,144.11,140.24,140.15,132.68,132.03,131.81,130.86,128.29,125.67,125.55,124.13,124.05,122.81,120.40,115.89,115.81,110.22,110.19,109.45,109.35,108.86,108.77,99.01,74.79,70.72,70.67,62.83,58.94,58.86,55.69,55.56,50.71,50.63,38.16,25.92,25.86.
[0091]
hrms m/z(esi)calcd for c
34h27
f3n2o6[m na]
639.1713;found 639.1725.
[0092]
实施例十四
[0093]
2,3-二氢-5-甲氧基-2-(1-萘亚甲基)-1h-茚酮代替实施例一中的2,3-二氢-5-甲
氧基-2-苯基亚甲基-1h-茚酮,反应结束后经纯化以62%产率和6:1dr值得到黄色固体产物,其熔点为144℃。
[0094]
产物3na的结构表征数据如下:
[0095]1h nmr(400mhz,cdcl3)δ8.06(d,j=7.3hz,1h),7.79(d,j=8.4hz,1h),7.68(t,j=10.3hz,4h),7.44(q,j=9.2,8.4hz,1h),7.26(ddt,j=30.6,14.7,7.1hz,4h),7.12(s,1h),6.89(d,j=8.5hz,1h),6.81(s,1h),6.56(d,j=7.7hz,1h),6.35(q,j=12.7,10.8hz,1h),6.16(s,1h),5.44(s,1h),4.67(d,j=10.3hz,1h),3.85(s,3h),3.63(s,4h),3.48-3.30(m,2h),2.90(s,3h).
[0096]
13
c nmr(101mhz,cdcl3)δ205.94,175.84,166.63,165.56,155.23,144.15,140.59,140.51,133.47,132.93,130.56,130.49,129.09,128.51,128.28,128.23,126.32,125.60,125.41,124.46,124.36,123.82,122.84,122.59,115.61,110.21,109.32,109.23,108.66,108.59,98.43,75.72,71.29,63.18,55.62,55.50,51.72,50.68,50.60,38.05,25.83,25.75.
[0097]
hrms m/z(esi)calcd for c
37h30
n2o5[m na]
605.2047;found 605.2054.
[0098]
实施例十五
[0099]
1-(2,3-二氢-1-乙基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐代替实施例一中的1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐,反应结束后经纯化以82%产率和10:1dr值得到黄色固体产物,其熔点为141℃。
[0100]
产物3ab的结构表征数据如下:
[0101]1h nmr(400mhz,cdcl3)δ7.83(dd,j=7.5,1.3hz,1h),7.69(d,j=8.5hz,1h),7.32(td,j=7.7,1.3hz,1h),7.19(td,j=7.6,1.0hz,1h),7.11(dd,j=5.1,2.0hz,3h),7.01(dq,j=4.8,2.5hz,3h),6.89(dd,j=8.5,2.3hz,1h),6.81(d,j=2.2hz,1h),6.72(d,j=7.8hz,1h),6.28(dt,j=10.3,1.8hz,1h),5.93(t,j=2.1hz,1h),4.61-4.53(m,1h),4.30(s,1h),3.85(s,3h),3.75-3.67(m,1h),3.58(s,3h),3.40-3.28(m,3h),0.90(t,j=7.4hz,4h).
[0102]
13
c nmr(101mhz,cdcl3)δ205.88,175.19,166.61,165.61,155.40,143.31,140.55,140.49,133.97,130.81,130.54,128.43,128.05,127.63,125.87,125.58,124.79,123.66,122.79,115.66,110.11,109.37,109.32,108.58,98.59,74.93,74.44,70.90,62.85,60.05,59.99,55.64,55.55,50.64,50.59,38.06,34.42,12.04.
[0103]
hrms m/z(esi)calcd for c
34h30
n2o5[m na]
569.2047;found 569.2050.
[0104]
实施例十六
[0105]
1-(2,3-二氢-1-苯基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐代替1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐,反应结束后经纯化以74%产率和》99:1dr值得到黄色固体产物,其熔点为139℃。
[0106]
产物3ac的结构表征数据如下:
[0107]1h nmr(400mhz,cdcl3)δ7.99-7.91(m,1h),7.70(d,j=8.5hz,1h),7.24-7.11(m,8h),7.08(d,j=7.2hz,2h),7.02(s,1h),6.90(dd,j=8.5,2.2hz,1h),6.82(d,j=2.2hz,1h),6.69-6.61(m,2h),6.53(dd,j=6.4,2.2hz,1h),6.27(dt,j=10.4,1.8hz,1h),6.03(t,j=2.1hz,1h),5.09(d,j=15.9hz,1h),4.60(dd,j=10.2,2.2hz,1h),4.41-4.28(m,
2h),3.85(s,3h),3.58(s,3h),3.32(s,2h).
[0108]
13
c nmr(101mhz,cdcl3)δ206.18,175.53,166.58,165.62,155.24,143.53,140.65,140.54,134.72,134.31,131.31,130.60,128.63,128.38,127.78,127.39,126.68,126.57,125.99,125.59,124.89,123.95,123.00,122.93,115.82,110.02,109.75,109.64,109.41,109.28,98.52,75.18,70.93,62.93,59.85,59.76,55.69,55.53,50.68,50.57,43.66,37.67.
[0109]
hrms m/z(esi)calcd for c
39h32
n2o5[m na]
631.2203;found 631.2205.
[0110]
实施例十七
[0111]
1-(2,3-二氢-1-异丙基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐代替实施例一中的1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐,反应结束后经纯化以68%产率和13:1dr值得到黄色固体产物,其熔点为146℃。
[0112]
产物3ad的结构表征数据如下:
[0113]1h nmr(400mhz,cdcl3)δ7.83(dd,j=7.5,1.3hz,1h),7.68(d,j=8.5hz,1h),7.29(dd,j=8.1,6.7hz,1h),7.17(t,j=7.6hz,1h),7.11(dd,j=5.2,2.0hz,3h),7.05(s,1h),7.03-6.96(m,2h),6.92-6.78(m,3h),6.27(dt,j=10.2,1.8hz,1h),5.94(t,j=2.2hz,1h),4.56(dd,j=10.4,2.1hz,1h),4.34-4.21(m,2h),3.83(s,3h),3.58(s,3h),3.34(d,j=3.4hz,2h),1.28(d,j=7.0hz,3h),1.13(d,j=7.0hz,3h).
[0114]
13
c nmr(101mhz,cdcl3)δ205.97,175.25,166.64,165.58,155.38,142.84,140.67,140.63,133.98,130.90,130.28,128.39,128.00,127.61,125.96,125.55,125.01,123.29,122.76,115.66,110.05,109.35,109.31,98.36,74.91,71.03,62.89,60.36,60.31,55.61,55.54,50.62,50.57,43.75,38.00,18.85.
[0115]
hrms m/z(esi)calcd for c
35h32
n2o5[m na]
583.2203;found 583.2208.
[0116]
实施例十八
[0117]
1-(5-氟-2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐代替实施例一中的1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐,反应结束后经纯化以46%产率和》99:1dr值得到黄色固体产物,其熔点为154℃。
[0118]
产物3ae的结构表征数据如下:
[0119]1h nmr(400mhz,cdcl3)δ7.69(d,j=8.6hz,1h),7.60(dd,j=7.6,2.6hz,1h),7.12(q,j=4.1,3.2hz,3h),7.07-6.97(m,3h),6.95(s,1h),6.89(dd,j=8.5,2.2hz,1h),6.79(d,j=2.2hz,1h),6.65(dd,j=8.6,4.0hz,1h),6.28(dt,j=10.3,1.8hz,1h),5.86(d,j=2.2hz,1h),4.57(dd,j=10.3,2.0hz,1h),4.28(s,1h),3.84(s,3h),3.59(s,3h),3.32(s,2h),2.98(s,3h).
[0120]
13
c nmr(101mhz,cdcl3)δ205.51,175.46,166.53,165.71,160.85,158.43,155.40,140.14,140.12,140.00,139.94,133.61,130.54,128.34,128.21,127.84,126.31,126.23,125.65,122.73,117.27,117.08,115.76,115.71,113.76,113.51,110.39,110.36,109.41,109.36,99.47,74.83,70.76,70.72,62.73,60.22,60.16,55.66,55.57,50.76,50.70,38.12,26.05,26.01.
[0121]
hrms m/z(esi)calcd for c
33h27
fn2o5[m na]
573.1796;found 573.1807.
[0122]
实施例十九
[0123]
1-(6-氯-2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐实施例一中的1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐,反应结束后经纯化以50%产率和3:1dr值得到黄色固体产物,其熔点为150℃。
[0124]
产物3af的结构表征数据如下:
[0125]1h nmr(400mhz,cdcl3)δ7.73(d,j=8.0hz,1h),7.68(d,j=8.5hz,1h),7.26(s,1h),7.12(dd,j=5.1,1.9hz,3h),6.99(dd,j=6.5,2.9hz,2h),6.94(s,1h),6.88(dd,j=8.5,2.2hz,1h),6.79(d,j=2.2hz,1h),6.72(d,j=1.9hz,1h),6.27(dt,j=10.2,1.8hz,1h),5.86(t,j=2.2hz,1h),4.57(dd,j=10.2,2.0hz,1h),4.28(s,1h),3.83(s,3h),3.58(s,3h),3.31(s,2h),2.97(s,3h).
[0126]
13
c nmr(101mhz,cdcl3)δ205.59,175.60,166.53,165.71,155.42,145.37,136.55,133.65,130.53,129.62,128.34,128.26,126.65,125.65,123.80,122.95,122.73,115.73,110.41,109.46,102.20,99.22,74.48,70.71,62.82,59.98,59.91,56.23,55.67,55.58,50.76,50.69,38.14,26.04.
[0127]
hrms m/z(esi)calcd for c
33h27
cln2o5[m na]
589.1501;found 589.1505.
[0128]
实施例二十
[0129]
1-(5-氯-2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐代替实施例一中的1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐,反应结束后经纯化以64%产率和51:1dr值得到黄色固体产物,其熔点为155℃。
[0130]
产物3ag的结构表征数据如下:
[0131]1h nmr(400mhz,cdcl3)δ7.69(d,j=8.5hz,1h),7.56-7.51(m,1h),7.10(dq,j=5.8,2.4hz,4h),7.04-6.96(m,3h),6.88(dd,j=8.5,2.3hz,1h),6.78(d,j=2.2hz,1h),6.60(d,j=8.0hz,1h),6.31-6.25(m,1h),5.86(t,j=2.1hz,1h),4.56(ddd,j=10.3,2.1,0.9hz,1h),4.34(s,1h),3.83(s,3h),3.58(s,3h),3.32(s,2h),3.00(s,3h),2.37(s,3h).
[0132]
13
c nmr(101mhz,cdcl3)δ205.74,175.54,166.67,165.64,155.62,141.81,140.43,140.33,133.94,133.59,131.06,130.98,130.40,128.48,128.13,127.57,125.98,125.57,124.27,122.67,115.70,115.61,110.25,110.21,109.38,109.28,108.46,108.37,98.95,74.65,70.56,70.51,62.84,59.79,59.71,55.65,55.51,50.67,50.58,38.48,25.95,25.88,21.19,21.09.
[0133]
hrms m/z(esi)calcd for c
34h30
n2o5[m na]
569.2047;found 569.2047.
[0134]
实施例二十一
[0135]
1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(乙酰基)-吡啶溴鎓盐代替实施例一中的1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐,反应结束后经纯化以58%产率和》99:1dr值得到黄色固体产物,其熔点为155℃。
[0136]
产物3ah的结构表征数据如下:
[0137]1h nmr(400mhz,cdcl3)δ7.80(d,j=7.4hz,1h),7.67(d,j=8.5hz,1h),7.35(td,j=7.7,1.3hz,1h),7.21(td,j=7.6,1.0hz,1h),7.11(dd,j=5.1,1.9hz,3h),7.03-6.94(m,3h),6.88(dd,j=8.5,2.2hz,1h),6.79(d,j=2.2hz,1h),6.73(d,j=7.8hz,1h),6.41(dt,j=10.5,1.9hz,1h),5.91(t,j=2.2hz,1h),4.64(dd,j=10.2,2.3hz,1h),4.33(s,1h),3.83(s,3h),3.33(s,2h),3.00(s,3h),1.94(s,3h).
[0138]
13
c nmr(101mhz,cdcl3)δ205.53,191.48,175.30,165.67,155.40,144.18,141.18,141.10,133.71,130.86,130.50,128.27,128.16,127.73,125.60,125.47,124.13,123.86,122.17,115.75,115.67,111.04,109.67,109.40,109.30,108.85,108.77,75.15,70.69,70.65,62.85,59.93,59.84,55.65,55.53,38.08,25.93,25.87,24.27.
[0139]
hrms m/z(esi)calcd for c
33h28
n2o4[m h]
517.2122;found 517.2123.
[0140]
实施例二十二
[0141]
1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(氰基)-吡啶溴鎓盐代替实施例一中的1-(2,3-二氢-1-甲基-2-氧代-1h-吲哚-3-基)-3-(甲氧羰基)-吡啶溴鎓盐,反应结束后经纯化以79%产率和》99:1dr值得到黄色固体产物,其熔点为157℃。
[0142]
产物3ai的结构表征数据如下:
[0143]1h nmr(400mhz,cdcl3)δ7.71(dd,j=13.3,8.0hz,2h),7.35(t,j=7.7hz,1h),7.21(t,j=7.6hz,1h),7.10(q,j=4.1,3.3hz,3h),7.00(dt,j=7.5,3.7hz,2h),6.89(dd,j=8.6,2.2hz,1h),6.79(d,j=2.2hz,1h),6.74(d,j=7.8hz,1h),6.49(s,1h),5.84(d,j=2.3hz,1h),5.77(d,j=9.9hz,1h),4.61(dd,j=10.1,2.1hz,1h),4.32(s,1h),3.83(s,3h),3.33(s,2h),3.04(s,3h).
[0144]
13
c nmr(101mhz,cdcl3)δ205.27,175.21,165.79,155.55,144.17,141.63,141.58,133.38,131.02,130.37,128.25,128.22,127.79,125.66,125.44,123.96,123.57,121.72,120.68,115.81,115.77,112.19,109.41,109.36,108.83,78.71,74.60,69.70,69.67,62.99,59.29,59.23,55.66,55.58,38.34,25.98,25.94.
[0145]
hrms m/z(esi)calcd for c
32h25
n3o3[m na]
522.1788;found 522.1790.
再多了解一些
本文用于创业者技术爱好者查询,仅供学习研究,如用于商业用途,请联系技术所有人。