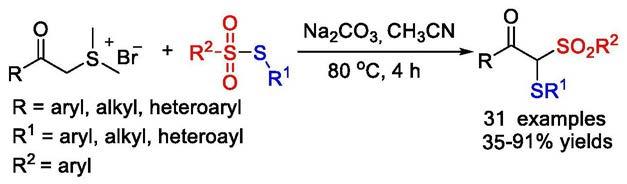
1.该专利涉及有机合成、药物合成、有机化工的研究领域,具体的方法是从芳甲酰亚甲基二甲基溴化硫和硫代磺酸酯的在碳酸钠的作用下进行双官能团化反应一步合成β-酮硫代砜类化合物。
背景技术:
2.砜类化合物是一种常见的有机中间体,在医药、农药以及新型功能材料等领域中都扮演着极其重要的角色。在医药方面,例如用于治疗抗偏头痛药物依来曲普坦、治疗乳腺癌的阿多醌、治疗骨关节炎的依他昔布(ye.s,yang.m,wu.j,chem.commun.2020,56,4145-4155.),以及用于用于预防阿尔茨海默氏病的药物γ-分泌酶抑制剂(i.churcher,d.beher,j.d.best,j.l.castro,e.e.clarke,a.gentry,t.harrison,l.hitzel,e.kay,s.kerrad,h.d.lewis,p.m.gutierrez,r.m.smith,p.j.oakley,m.reilly,d.e.shaw,m.s.shearman,m.r.teall,s.williams and j.d.j.wrigley,bioorg.med.chem.lett.,2006,16,280.)均含有砜的单元结构。在有机农药方面,砜类化合物具有很好的抗菌活性,例如除草剂唑草胺(g.mitchell,d.w.bartlett,t.e.m.fraser,t.r.hawkes,d.c.holt,j.k.townson,r.a.wichert,pest manag.sci.2001,57,120-128.)、异唑草酮(s.taylor-lovell,g.k.sims,l.m.wax,j.agr.food.chem.2002,50,5626-5633.)、杀虫剂氧化萎锈灵(k.hustert,p.n.moza,a.kettrup,chemosphere.1999,38,3423-3429.)。在新型功能材料方面,多元二芳砜分子具有特殊的光物理性质,在发光二极管材料中具有广阔的用途(g.barbarella,l.favaretto,a.zanelli,g.gigli,g.m.mazzeo,m.anni,a.bongini,adv.funct.mater.2005,15,664-670.)。砜也常被用作有机合成的重要中间体(n.s.simpkins,sulfones in organic synthesis;pergamon press:oxford,1993.)。基于此重要性,拓展新的砜类化合物的合成方法具有极大的价值。
3.合成β-酮硫代砜主要有以下几种方法:(1)通过铜/二联吡啶配合物催化重氮乙酸芳基酯和硫叶立德的stevens重排反应(h.yuan,t.nuligonda,h.gao,c.h.tung,z.xu,org.chem.front.2018,5,1371-1374.)。(2)铜催化烯烃的氧化三官能化反应(s.huang,n.thirupathi,c.h.tung,z.xu,j.org.chem.2018,83,9449-9455.)。(3)硫代磺酸盐和氧硫叶立德的串联自由基反应(f.wang,b.x.liu,w.rao,s.y.wang,org.lett.2020,22,6600-6604.)。上述各种合成β-酮硫代砜的方法存在需要金属催化,原料不易制备、操作繁琐等缺点,因此开发高效、便捷的合成砜类化合物的新方法具有极大的意义。
4.我们在此报告了一种芳甲酰亚甲基二甲基溴化硫和硫代磺酸酯的双官能团化反应,开发了一种新的β-酮硫代砜类化合物的合成方法。
5.尽我们所知,未见与本技术相同的文献报道。
技术实现要素:
6.本发明提供β-酮硫代砜类化合物的合成方法。
7.本发明公开的β-酮硫代砜类化合物合成方法均一步完成,即在乙腈的溶液条件下,以碳酸钠为碱,芳甲酰亚甲基二甲基溴化硫与硫代磺酸酯发生双官能团化反应一步合成β-酮硫代砜类化合物,反应方程式如下所示。
[0008][0009]
结合下面的实施例,更详细地阐述本发明,但并不认为它们是对本发明范围的限制。
具体实施方式
[0010]
实施例一
[0011]
向装有搅拌子的25ml玻璃试管中加入苯甲酰亚甲基二甲基溴化硫(0.6mmol),s-苯基硫代苯磺酸酯(0.5mmol,碳酸钠(4当量),5ml乙腈,最后用橡皮塞密封磨口试管。在氮气氛围下用预热的80℃油浴锅将试管搅拌4h,之后将反应混合物冷却至室温。然后向反应混合物中加入饱和氯化钠溶液(10ml)淬灭,用乙酸乙酯(15ml)萃取3次,并将合并的有机层用无水mgso4干燥,然后在旋转蒸发仪上用碱性氧化铝吸附干燥。残余物通过硅胶快速柱色谱纯化(石油醚:乙酸乙酯=10∶1-5:1用作洗脱剂),纯化后,得到1-苯基-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮为白色固体,产率为82%。
[0012]
产物1-苯基-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮的结构表征数据如下:
[0013]1h nmr(400mhz,chloroform-d)δ8.05
–
7.97(m,2h),7.91
–
7.83(m,2h),7.70
–
7.64(m,1h),7.63
–
7.57(m,1h),7.57
–
7.41(m,6h),7.37
–
7.22(m,3h),5.82(s,1h).
[0014]
13
c nmr(101mhz,chloroform-d)δ189.3,136.3,135.1,134.5,134.4,133.6,132.1,130.7,129.5,129.4,129.2,128.9,128.7,75.6.
[0015]
hrms(esi-tof):m/z[m na]
calcd for c
20h16
nao3s
2
:391.0433;found:391.0443.
[0016]
实施例二
[0017]
4-(氟)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到白色固体1-(4-氟苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮为88%。
[0018]1h nmr(400mhz,chloroform-d)δ7.91(d,j=7.4hz,2h),7.88
–
7.81(m,2h),7.59(t,j=7.5hz,1h),7.46(t,j=7.8hz,2h),7.42
–
7.36(m,2h),7.22(tq,j=12.7,6.7,5.9hz,3h),7.04(t,j=8.5hz,2h),5.66(s,1h).
[0019]
13
c nmr(101mhz,chloroform-d)δ187.8,166.5(d,j=257.8hz),136.2,134.6,133.6,132.2(d,j=9.7hz),132.0,131.5(d,j=2.9hz),130.7,129.5(d,j=5.5hz),128.8,116.2(d,j=22.2hz),75.9.
[0020]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
fnao3s
2
:409.0339;found:409.0370.
[0021]
实施例三
[0022]
4-(氯)苯甲酰亚甲基二甲基溴化硫代替代替实施例一中的苯甲酰亚甲基二甲基
溴化硫,得到黄色油1-(4-氯苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为75%。
[0023]1h nmr(400mhz,chloroform-d)δ8.03
–
7.97(m,2h),7.86
–
7.81(m,2h),7.72
–
7.66(m,1h),7.55(t,j=7.8hz,2h),7.45(ddd,j=16.5,7.3,1.5hz,4h),7.37
–
7.27(m,3h),5.72(s,1h).
[0024]
13
c nmr(101mhz,chloroform-d)δ188.2,141.1,136.2,134.6,133.6,133.4,131.9,130.7,129.6,129.5,129.3,128.8,128.6,75.9.
[0025]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
clnao3s
2
:425.0043;found:425.0060.
[0026]
实施例四
[0027]
4-(溴)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到白色固体1-(4-溴苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为75%。
[0028]1h nmr(400mhz,chloroform-d)δ7.96
–
7.87(m,2h),7.69
–
7.63(m,2h),7.62
–
7.56(m,1h),7.54
–
7.43(m,4h),7.41
–
7.35(m,2h),7.22(ddd,j=12.7,7.9,6.1hz,3h),5.64(s,1h).
[0029]
13
c nmr(101mhz,chloroform-d)δ188.4,136.2,134.6,133.8,133.6,132.3,131.8,130.7,130.7,130.0,129.6,129.6,128.8,75.8.
[0030]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
brnao3s
2
:468.9538;found:468.9535
[0031]
实施例五
[0032]
4-(氰基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到4-(2-(苯磺酰基)-2-(苯硫基)乙酰基)苄腈产率为62%。
[0033]1h nmr(400mhz,chloroform-d)δ8.03
–
7.97(m,4h),7.80
–
7.68(m,3h),7.58(t,j=7.7hz,2h),7.47
–
7.41(m,2h),7.39
–
7.25(m,3h),5.72(s,1h).
[0034]
13
c nmr(101mhz,chloroform-d)δ187.2,136.9,135.0,133.8,132.6,131.6,130.5,130.4,129.6,128.7,128.6,127.9,116.6,116.4,74.9.
[0035]
hrms(esi-tof):m/z[m na]
calcd for c
21h15
nnao3s
2
:416.0386;found:416.0378.
[0036]
实施例六
[0037]
4-(硝基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到红色固体1-(4-硝基苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为63%。
[0038]1h nmr(400mhz,chloroform-d)δ7.95
–
7.88(m,2h),7.87
–
7.81(m,2h),7.59(td,j=7.3,1.3hz,1h),7.46(t,j=7.9hz,2h),7.41
–
7.36(m,2h),7.28
–
7.16(m,3h),7.09
–
6.99(m,2h),5.67(s,1h).
[0039]
13
c nmr(101mhz,chloroform-d)δ187.8,167.8,165.2,136.2,134.6,133.6,132.2(d,j=9.8hz),132.0,131.5(d,j=3.0hz),130.7,129.5(d,j=5.4hz),128.8,116.2(d,j=22.1hz),75.9.
[0040]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
nnao5s
2
:436.0284;found:436.0278.
[0041]
实施例七
[0042]
4-(三氟甲基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到黄色固体2-(苯磺酰基)-2-(苯硫基)-1-(4-(三氟甲基)苯基)乙烷-1-酮产率为61%。
[0043]1h nmr(400mhz,chloroform-d)δ8.05
–
7.98(m,4h),7.76
–
7.67(m,3h),7.57(dd,j=8.4,7.2hz,2h),7.50
–
7.43(m,2h),7.39
–
7.27(m,3h),5.76(s,1h).
[0044]
13
c nmr(101mhz,chloroform-d)δ188.5,137.7,136.2,135.5,135.2,134.7,133.6,131.6,130.7,129.7,129.6,128.9,125.98,125.95,125.9,125.9,75.9.
[0045]
hrms(esi-tof):m/z[m na]
calcd for c
21h15
f3nao3s
2
:459.0307;found:459.0328.
[0046]
实施例八
[0047]
4-(三氟甲氧基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到白色固体2-(苯磺酰基)-2-(苯硫基)-1-(4-(三氟甲氧基)苯基)乙烷-1-酮产率为75%。
[0048]1h nmr(400mhz,chloroform-d)δ8.07
–
7.96(m,4h),7.71(t,j=7.5hz,1h),7.58(t,j=7.8hz,2h),7.53
–
7.47(m,2h),7.41
–
7.27(m,5h),5.78(s,1h).
[0049]
13
c nmr(101mhz,chloroform-d)δ187.9,153.5(d,j=1.7hz),136.2,134.6,133.6,133.1,131.9,131.5,130.7,129.6(d,j=2.8hz),128.8,121.5,120.4,119.0,75.9.
[0050]
hrms(esi-tof):m/z[m na]
calcd for c
21h15
f3nao4s
2
:475.0256;found:475.0249.
[0051]
实施例九
[0052]
4-(甲氧基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到黄色油1-(4-甲氧基苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为64%。
[0053]1h nmr(400mhz,chloroform-d)δ8.01
–
7.96(m,2h),7.91
–
7.85(m,2h),7.65(d,j=7.5hz,1h),7.58
–
7.44(m,4h),7.35
–
7.25(m,3h),6.97
–
6.87(m,2h),5.78(s,1h),3.86(s,3h).
[0054]
13
c nmr(101mhz,chloroform-d)δ187.6,164.7,136.4,134.4,133.5,132.4,131.9,130.7,129.5,129.3,128.7,128.0,114.2,75.7,55.7.
[0055]
hrms(esi-tof):m/z[m na]
calcd for c
21h18
nao4s
2
:421.0539;found:421.0560.
[0056]
实施例十
[0057]
4-(苯基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到白色固体1-([1,1'-联苯]-4-基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为91%。
[0058]1h nmr(400mhz,chloroform-d)δ8.09
–
8.04(m,2h),8.03
–
7.97(m,2h),7.74
–
7.69(m,3h),7.65(dt,j=8.3,1.9hz,2h),7.62
–
7.54(m,4h),7.54
–
7.48(m,2h),7.47
–
7.42(m,1h),7.41
–
7.32(m,3h),5.87(s,1h).
[0059]
13
c nmr(101mhz,chloroform-d)δ188.8,147.1,139.4,136.3,134.5,133.7,133.6,132.3,130.8,129.9,129.5,129.4,129.1,128.7,128.7,127.5,127.4,75.8.
[0060]
hrms(esi-tof):m/z[m na]
calcd for c
26h20
nao3s
2
:467.0746;found:467.0747.
[0061]
实施例十一
[0062]
3-(溴)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到白色固体1-(3-溴苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为85%。
[0063]1h nmr(400mhz,chloroform-d)δ8.01(d,j=7.5hz,2h),7.96(t,j=1.6hz,1h),7.80(d,j=7.9hz,1h),7.75
–
7.66(m,2h),7.57(t,j=7.8hz,2h),7.51
–
7.47(m,2h),7.40
–
7.29(m,4h),5.70(s,1h).
[0064]
13
c nmr(101mhz,chloroform-d)δ188.1,137.2,136.8,136.2,134.7,133.7,132.0,131.7,130.7,130.4,129.7,129.6,128.8,127.8,123.2,75.7.
[0065]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
brnao3s
2
:468.9538;found:468.9544.
[0066]
实施例十二
[0067]
3-(氯)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到无色油1-(3-氯苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为84%。
[0068]1h nmr(400mhz,chloroform-d)δ8.01(dd,j=8.4,1.1hz,2h),7.81(t,j=1.8hz,1h),7.76(dt,j=7.8,1.3hz,1h),7.69(tt,j=7.1,1.2hz,1h),7.56(td,j=7.1,6.2,1.6hz,3h),7.51
–
7.45(m,2h),7.41(d,j=7.9hz,1h),7.39
–
7.32(m,2h),7.32
–
7.27(m,1h),5.70(s,1h).
[0069]
13
c nmr(101mhz,chloroform-d)δ188.2,136.6,136.2,135.3,134.7,134.3,133.7,131.8,130.7,130.2,129.6,129.6,129.1,128.8,127.3,75.7.
[0070]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
clnao3s
2
:425.0043;found:425.0025.
[0071]
实施例十三
[0072]
3-(甲氧基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到黄色油1-(3-甲氧基苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率产率为63%。
[0073]1h nmr(400mhz,chloroform-d)δ8.09
–
8.01(m,2h),7.69(d,j=7.4hz,1h),7.61
–
7.50(m,4h),7.45(d,j=7.7hz,1h),7.42
–
7.29(m,5h),7.21
–
7.15(m,1h),5.81(s,1h),3.82(s,3h).
[0074]
13
c nmr(101mhz,chloroform-d)δ189.1,160.0,136.4(d,j=0.9hz),134.5,133.6,132.2,130.8,129.9,129.5,129.4,128.7,121.8,121.3,113.1,75.6,55.5.
[0075]
hrms(esi-tof):m/z[m na]
calcd for c
21h18
nao4s
2
:421.0539;found:421.0532.
[0076]
实施例十四
[0077]
2-(甲氧基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到白色固体1-(2-甲氧基苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为45%。
[0078]1h nmr(400mhz,chloroform-d)δ8.07
–
8.00(m,2h),7.71
–
7.63(m,2h),7.60
–
7.46(m,5h),7.33(dd,j=4.9,1.7hz,3h),7.02(t,j=7.5hz,1h),6.87(d,j=8.4hz,1h),6.50(s,1h),3.54(s,3h).
[0079]
13
c nmr(101mhz,chloroform-d)δ190.2,158.5,137.1,135.3,134.2,133.5,132.6,131.8,130.6,129.2,128.7,128.5,125.8,121.3,111.7,78.2,55.4.
[0080]
hrms(esi-tof):m/z[m na]
calcd for c
21h18
nao4s
2
:421.0539;found:421.0538.
[0081]
实施例十五
[0082]
2,5-(二甲氧基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到黄色油1-(2,5-二甲氧基苯基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为58%。
[0083]1h nmr(400mhz,chloroform-d)δ8.05
–
7.98(m,2h),7.65(tt,j=6.9,1.2hz,1h),7.58
–
7.50(m,4h),7.34
–
7.29(m,3h),7.21
–
7.14(m,1h),7.05(dd,j=9.1,3.3hz,1h),6.80(d,j=9.1hz,1h),6.57(s,1h),3.76(s,3h),3.48(s,3h).
[0084]
13
c nmr(101mhz,chloroform-d)δ189.7,153.7,153.2,137.1,134.2,133.4,132.6,130.7,129.2,128.7,128.5,125.7,122.5,114.5,113.3,78.0(d,j=3.5hz),55.8(d,j=5.6hz).
[0085]
hrms(esi-tof):m/z[m na]
calcd for c
22h20
nao5s
2
:451.0644;found:451.0638.
[0086]
实施例十六
[0087]
1-(萘基)苯甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到白色固体1-(萘基)-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为64%。
[0088]1h nmr(400mhz,chloroform-d)δ8.34(s,1h),8.09
–
8.02(m,2h),7.95
–
7.91(m,1h),7.89
–
7.82(m,3h),7.63(dt,j=15.9,7.4hz,2h),7.54(t,j=7.7hz,5h),7.38
–
7.27(m,3h),5.96(s,1h).
[0089]
13
c nmr(101mhz,chloroform-d)δ189.2,136.4,136.0,134.5,133.8,132.4,132.3,131.7,130.8,130.0,129.6,129.5,129.5,128.9,128.8,127.8,127.2,124.1,75.8.
[0090]
hrms(esi-tof):m/z[m na]
calcd for c
24h18
nao3s
2
:441.0590;found:441.0582.
[0091]
实施例十七
[0092]
环丙基甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到无色油1-环丙基-2-(苯磺酰基)-2-(苯硫基)乙烷-1-酮产率为68%。
[0093]1h nmr(400mhz,chloroform-d)δ8.00
–
7.94(m,2h),7.68(t,j=7.5hz,1h),7.55(t,j=7.8hz,2h),7.42
–
7.37(m,2h),7.33
–
7.23(m,3h),4.93(s,1h),2.44
–
2.33(m,1h),1.19
–
1.07(m,4h).
[0094]
13
c nmr(101mhz,chloroform-d)δ198.8,136.9,134.6,133.2,131.8,129.8,129.5,129.2,129.0,80.6,20.8,14.1,13.7.
[0095]
hrms(esi-tof):m/z[m na]
calcd for c
17h16
nao3s
2
:355.0433;found:355.0430.
[0096]
实施例十八
[0097]
噻吩甲酰亚甲基二甲基溴化硫代替实施例一中的苯甲酰亚甲基二甲基溴化硫,得到无色油2-(苯磺酰基)-2-(苯硫基)-1-(噻吩-2-基)乙烷-1-酮产率为65%。
[0098]1h nmr(400mhz,chloroform-d)δ8.02
–
7.96(m,2h),7.74(dd,j=7.9,4.4hz,2h),7.67(t,j=7.5hz,1h),7.57
–
7.48(m,4h),7.37
–
7.27(m,3h),7.16
–
7.10(m,1h),5.57(s,1h).
[0099]
13
c nmr(101mhz,chloroform-d)δ181.7,142.1,136.7,136.1,134.8,134.6,133.6,132.4,130.7,129.5,129.4,128.8,128.7,77.4.
[0100]
hrms(esi-tof):m/z[m na]
calcd for c
18h14
nao3s
3
:396.9997;found:396.9978
[0101]
实施例十九
[0102]
s-(4-氯苯基)-硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色固体2-((4-氯苯基)硫代)-1-苯基-2-(苯磺酰基)乙烷-1-酮产率为77%。
[0103]1h nmr(400mhz,chloroform-d)δ7.98
–
7.93(m,2h),7.88(dd,j=8.4,1.1hz,2h),7.64
–
7.58(m,1h),7.55
–
7.49(m,4h),7.49
–
7.42(m,2h),7.38
–
7.28(m,3h),5.85(s,1h).
[0104]
13
c nmr(101mhz,chloroform-d)δ189.3,141.5,135.0 134.6,134.6,133.6,132.4,131.9,129.8,129.6,129.3,129.1,129.0,75.3,75.3.
[0105]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
clnao3s
2
:425.0043;found:425.0046.
[0106]
实施例二十
[0107]
s-(4-甲基苯基)-硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色固体2-((4-甲基苯基)硫代)-1-苯基-2-(苯磺酰基)乙烷-1-酮产率为61%。
[0108]1h nmr(400mhz,chloroform-d)δ8.04
–
7.98(m,2h),7.90
–
7.84(m,2h),7.67(tt,j=7.0,1.2hz,1h),7.64
–
7.52(m,3h),7.45(td,j=7.5,1.6hz,2h),7.40
–
7.35(m,2h),7.10(d,j=7.9hz,2h),5.74(s,1h),2.33(s,3h).
[0109]
13
c nmr(101mhz,chloroform-d)δ189.3,140.0,136.5,135.1,134.5,134.4,134.2,130.8,130.3,129.2,128.9,128.7,128.4,75.8(d,j=5.3hz),21.32(d,j=6.0hz).
[0110]
hrms(esi-tof):m/z[m na]
calcd for c
21h18
nao3s
2
:405.0590;found:405.0596.
[0111]
实施例二十一
[0112]
s-(4-甲氧基苯基)-硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色固体2-((4-甲氧基苯基)硫代)-1-苯基-2-(苯磺酰基)乙烷-1-酮产率为69%。
[0113]1h nmr(400mhz,chloroform-d)δ8.06
–
8.00(m,2h),7.88
–
7.83(m,2h),7.67(t,j=7.5hz,1h),7.57(dt,j=21.1,7.5hz,3h),7.48
–
7.37(m,4h),6.80(d,j=8.8hz,2h),5.68(s,1h),3.78(s,3h).
[0114]
13
c nmr(101mhz,chloroform-d)δ184.5 156.3,131.9,130.4,129.7,129.6,125.9,124.4,124.2,124.0,117.2,110.2,71.2,50.7.
[0115]
hrms(esi-tof):m/z[m na]
calcd for c
21h18
nao4s
2
:421.0539;found:421.0541.
[0116]
实施例二十二
[0117]
s-(2-氟苯基)-硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色固体2-((2-氟苯基)硫代)-1-苯基-2-(苯磺酰基)乙烷-1-酮产率为60%。
[0118]1h nmr(400mhz,chloroform-d)δ7.95(ddd,j=19.3,8.4,1.1hz,4h),7.69
–
7.60(m,2h),7.60
–
7.48(m,5h),7.40
–
7.32(m,1h),7.17
–
7.03(m,2h),6.02(d,j=0.7hz,1h).
[0119]
13
c nmr(101mhz,chloroform-d)δ188.4,162.5,160.0,134.9,134.7,134.2,133.5(d,j=4.2hz),130.8(d,j=8.2hz),129.5,128.3,127.9,127.7,124.0(d,j=3.8hz),117.8(d,j=17.3hz),115.2(d,j=22.6hz),72.6(d,j=2.7hz).
[0120]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
fnao3s
2
:409.0339;found:409.0349.
[0121]
实施例二十三
[0122]
s-丙基硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到黄色固体1-苯
基-2-(苯磺酰基)-2-(丙硫基)乙烷-1-酮产率为65%。
[0123]1h nmr(400mhz,chloroform-d)δ8.03
–
7.90(m,4h),7.72
–
7.58(m,2h),7.55(t,j=7.8hz,2h),7.48(t,j=7.8hz,2h),5.59(s,1h),2.87(ddd,j=12.1,8.1,6.5hz,1h),2.72(ddd,j=12.1,7.9,7.0hz,1h),1.57(dt,j=15.2,7.8hz,2h),0.92(t,j=7.3hz,3h).
[0124]
13
c nmr(101mhz,chloroform-d)δ189.1,136.3,135.0,134.4,134.4,130.6,129.0,129.0,128.7,70.6(d,j=6.8hz),34.7,22.4,13.3.
[0125]
hrms(esi-tof):m/z[m na]
calcd for c
17h18
nao3s
2
:357.0590;found:357.0590.
[0126]
实施例二十四
[0127]
s-丁基硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色固体1-苯基-2-(苯磺酰基)-2-(丙硫基)乙烷-1-酮产率为66%。
[0128]1h nmr(400mhz,chloroform-d)δ8.04
–
7.92(m,4h),7.63(dt,j=21.2,7.4hz,2h),7.54(t,j=7.8hz,2h),7.47(t,j=7.8hz,2h),5.61(s,1h),2.88(ddd,j=12.0,8.2,6.5hz,1h),2.73(ddd,j=12.0,8.1,7.0hz,1h),1.60
–
1.44(m,2h),1.32(h,j=7.2hz,2h),0.85(t,j=7.3hz,3h).
[0129]
13
c nmr(101mhz,chloroform-d)δ189.1,136.4,135.0,134.4,134.3,130.6,129.0,128.9,128.7,70.7,32.4,30.9,21.8,13.6.
[0130]
hrms(esi-tof):m/z[m na]
calcd for c
18h20
nao3s
2
:371.0746;found:371.0749.
[0131]
实施例二十五
[0132]
s-十二烷基硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色固体2-(十二硫基)-1-苯基-2-(苯磺酰基)乙烷-1-酮产率为65%。
[0133]1h nmr(400mhz,chloroform-d)δ8.00(d,j=7.4hz,2h),7.96(d,j=7.4hz,2h),7.64(dt,j=21.3,7.4hz,2h),7.54(t,j=7.8hz,2h),7.47(t,j=7.8hz,2h),5.60(s,1h),2.87(ddd,j=12.1,8.1,6.6hz,1h),2.78
–
2.68(m,1h),1.58
–
1.44(m,2h),1.24(t,j=14.6hz,18h),0.88(t,j=6.8hz,3h).
[0134]
13
c nmr(101mhz,chloroform-d)δ189.1,136.4,135.1,134.3,134.3,130.6,129.0,128.9,128.6,70.7,32.8,31.9,29.6,29.5,29.4,29.35,29.1,28.8,28.6,22.7,14.2.
[0135]
hrms(esi-tof):m/z[m na]
calcd for c
26h36
nao3s
2
:483.1998;found:483.2004.
[0136]
实施例二十六
[0137]
s-吡啶基硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到红色油2-((4-溴苯基)磺酰基)-1-苯基-2-(吡啶-2-基硫基)乙烷-1-酮产率为50%。
[0138]1h nmr(400mhz,chloroform-d)δ8.37(ddd,j=4.9,1.7,0.9hz,1h),8.14
–
8.07(m,2h),7.96(dt,j=8.6,1.7hz,2h),7.71(s,1h),7.63
–
7.57(m,1h),7.57
–
7.50(m,1h),7.50
–
7.40(m,5h),7.16
–
7.11(m,1h),7.04(ddd,j=7.3,4.9,0.9hz,1h).
[0139]
13
c nmr(101mhz,chloroform-d)δ189.7,152.9,149.3,137.1,137.0,135.4,134.3,134.2,130.3,129.4,128.8,128.9,122.2,121.2,67.5.
[0140]
hrms(esi-tof):m/z[m na]
calcd for c
19h15
nnao3s
2
:392.0386;found:392.0381.
[0141]
实施例二十七
[0142]
se-苯基硒代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色固体1-苯基-2-(苯基硒基)-2-(苯基磺酰基)乙烷-1-酮产率为35%。
[0143]1h nmr(400mhz,chloroform-d)δ8.09
–
8.03(m,2h),7.80(d,j=7.4hz,2h),7.67(t,j=7.4hz,1h),7.58(dq,j=15.6,7.5hz,5h),7.41(dt,j=15.5,7.6hz,3h),7.29(t,j=7.5hz,2h),5.82(s,1h).
[0144]
13
c nmr(101mhz,chloroform-d)δ189.4,137.1,135.8,135.0,134.3,134.2,130.6,129.7,129.5,128.9,128.8,128.7,127.7,68.1.
[0145]
hrms(esi-tof):m/z[m na]
calcd for c
20h16
nao3sse
:438.9578;found:438.9561.
[0146]
实施例二十八
[0147]
s-苯基-4-甲基硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色油1-苯基-2-(苯硫基)-2-甲苯磺酸-1-酮产率为80%。
[0148]1h nmr(400mhz,chloroform-d)δ7.88(dd,j=7.8,4.4hz,4h),7.59(d,j=7.4hz,1h),7.53
–
7.48(m,2h),7.44(t,j=7.8hz,2h),7.35
–
7.28(m,5h),5.82(s,1h),2.43(s,3h).
[0149]
13
c nmr(101mhz,chloroform-d)δ189.5,145.7,135.2,134.4,133.5,133.3,132.3,130.8,129.5,129.4,129.3,129.3,128.9,75.5,21.8.
[0150]
hrms(esi-tof):m/z[m na]
calcd for c
21h18
clnao3s
2
:405.0590;found:405.0600.
[0151]
实施例二十九
[0152]
s-苯基-4-氟硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色固体2-((4-氟苯基)磺酰基)-1-苯基-2-(苯硫基)乙烷-1-酮产率为70%。
[0153]1h nmr(400mhz,chloroform-d)δ8.03(ddd,j=10.0,5.1,2.5hz,2h),7.90
–
7.84(m,2h),7.64
–
7.58(m,1h),7.53(dt,j=6.6,1.5hz,2h),7.49
–
7.42(m,2h),7.36
–
7.28(m,3h),7.26
–
7.17(m,2h),5.85(s,1h).
[0154]
13
c nmr(101mhz,chloroform-d)δ189.4,167.7,165.2,135.0,134.6,133.9,133.8,133.5,132.1(d,j=3.1hz),131.9,129.6(d,j=4.4hz),129.2,129.0,116.2,115.9,75.3.
[0155]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
fnao3s
2
:409.0339;found:409.0339.
[0156]
实施例三十
[0157]
s-苯基-4-氯硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到黄色油2-((4-氯苯基)磺酰基)-1-苯基-2-(苯硫基)乙烷-1-酮产率为67%。
[0158]1h nmr(400mhz,chloroform-d)δ7.95(d,j=8.5hz,2h),7.88(d,j=7.6hz,2h),7.62(t,j=7.4hz,1h),7.57
–
7.49(m,4h),7.46(t,j=7.7hz,2h),7.33(dt,j=14.1,6.8hz,3h),5.84(s,1h).
[0159]
13
c nmr(101mhz,chloroform-d)δ189.3,141.5,135.0,134.6,134.6,133.6,132.3,131.9,129.6,129.6,129.2,129.0,75.3.
[0160]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
clnao3s
2
:425.0043;found:
425.0063.
[0161]
实施例三十一
[0162]
s-苯基-4-溴硫代苯磺酸酯代替实施例一中的s-苯基硫代苯磺酸酯,得到白色固体2-((4-溴苯基)磺酰基)-1-苯基-2-(苯硫基)乙烷-1-酮产率为66%。
[0163]1h nmr(400mhz,chloroform-d)δ7.92
–
7.83(m,4h),7.72
–
7.67(m,2h),7.63(t,j=7.4hz,1h),7.55
–
7.51(m,2h),7.47(t,j=7.8hz,2h),7.38
–
7.29(m,3h),5.82(s,1h).
[0164]
13
c nmr(101mhz,chloroform-d)δ189.2,135.1,135.0,134.6,133.6,132.4,132.0,131.9,130.2,129.59,129.55,129.2,129.0,75.4.
[0165]
hrms(esi-tof):m/z[m na]
calcd for c
20h15
brnao3s
2
:468.9538;found:468.9549.
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。