n
‑
酰基苯并咪唑类化合物的合成方法
技术领域
1.本发明属于有机合成技术领域,具体涉及n
‑
酰基苯并咪唑类化合物的合成方法。
背景技术:
2.n
‑
酰基苯并咪唑是一种温和的酰化试剂,在外消旋体的动力学拆分中具有重要且广泛用途。另外,n
‑
酰基苯并咪唑类化合物为cb2大麻素的有效受体,有望用于治疗痴呆、多发性硬化症和阿尔茨海默症等神经炎症性疾病,在合成化学及药物化学等领域具有重要的应用价值。
3.然而,该类化合物的合成方法仍很有限,而且这些文献方法主要是在预先制备好的苯并咪唑结构单元上进行n
‑
酰基结构修饰,尚存在原料不易得到、反应条件苛刻、官能团耐受性差等问题。
4.因此,研究并开发从价廉易得的原料出发、在相对温和的反应条件下合成n
‑
酰基苯并咪唑类化合物的新方法,具有重要的研究意义。
技术实现要素:
5.本发明主要提供了一种n
‑
酰基苯并咪唑类化合物新的合成方法,通过n
‑
芳基脒类化合物和二噁唑酮之间的一锅串联反应,高效合成n
‑
酰基苯并咪唑类化合物。该合成方法具有原料简单易得、操作简便、底物适用范围广等优点。
6.本发明所合成的n
‑
酰基苯并咪唑类化合物,其结构通式为:
[0007][0008]
其中,r1为氢、c1‑6烷基、c1‑4烷氧基、苯基、取代苯基、硝基或卤素,r2为苯基、取代苯基、噻吩基、呋喃基、萘基、c1‑8烷基、苯基取代c1‑3烷基或取代苯基取代c1‑3烷基,以上所述取代苯基苯环上的取代基为c1‑4烷基、c1‑4烷氧基、甲叉二氧基、三氟甲基、苯基或卤素。
[0009]
本发明还提供了上述n
‑
酰基苯并咪唑类化合物的合成方法,采用的技术方案为:
[0010]
n
‑
酰基苯并咪唑类化合物的合成方法,包括如下操作:将n
‑
芳基脒类化合物1、铑或钴催化剂、添加剂、二噁唑酮类化合物2和有机溶剂混合,升温反应得到n
‑
酰基苯并咪唑类化合物3。
[0011]
反应方程式为:
[0012][0013]
其中,r1为氢、c1‑6烷基、c1‑4烷氧基、苯基、取代苯基、硝基或卤素,r2为苯基、取代苯
基、噻吩基、呋喃基、萘基、c1‑8烷基、苯基取代c1‑3烷基或取代苯基取代c1‑3烷基,以上所述取代苯基苯环上的取代基为c1‑4烷基、c1‑4烷氧基、甲叉二氧基、三氟甲基、苯基或卤素。
[0014]
进一步地,在上述技术方案中,所述有机溶剂为起到溶解原料的作用,优选1,2
‑ꢀ
二氯乙烷、乙酸乙酯、1,4
‑
二氧六环、四氢呋喃或甲苯。
[0015]
进一步地,在上述技术方案中,所述铑催化剂为二氯(五甲基环戊二烯基)合铑(iii) 二聚体([rhcp*cl2]2),钴催化剂为五甲基环戊二烯基羰基二碘化钴(cocp*(co)i2)。
[0016]
进一步地,在上述技术方案中,所述添加剂采用银盐添加剂与醋酸盐添加剂中的一种或两种。该两种添加剂可单独用于反应,也可组合在一起用于反应。优选反应条件下,采用银盐添加剂和醋酸盐添加剂组成的混合添加剂。
[0017]
进一步地,在上述技术方案中,所述银盐添加剂为六氟锑酸银、三氟甲烷磺酸银、双三氟甲烷磺酰亚胺银或三氟乙酸银。
[0018]
进一步地,在上述技术方案中,所述醋酸盐添加剂为醋酸钠、醋酸铜、醋酸铯或醋酸锌。
[0019]
进一步地,在上述技术方案中,所述n
‑
芳基脒类化合物1、二噁唑酮2、铑或钴催化剂、银盐添加剂与醋酸盐添加剂摩尔比为1
‑
1.2:1
‑
1.2:0.02
‑
0.05:0.05
‑
0.2:0.1
‑
0.5。
[0020]
进一步地,在上述技术方案中,所述反应温度为80
‑
120℃。
[0021]
进一步地,在上述技术方案中,所述反应在空气氛围下进行。
[0022]
发明有益效果:
[0023]
本发明与现有技术相比具有以下优点:1)通过n
‑
芳基脒类化合物和二噁唑酮的一锅串联反应,即可在构建咪唑环的同时引入n
‑
酰基,直接合成出n
‑
酰基苯并咪唑类化合物,合成过程简单、高效;2)原料价廉易得,无需氧化剂,操作简便,底物的适用范围广。
附图说明
[0024][0025]
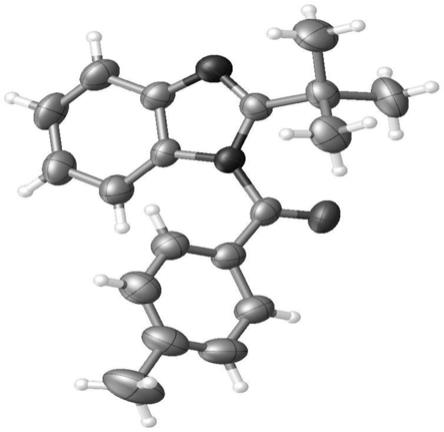
图1为实施例3中化合物3o的x
‑
射线单晶衍射图。
具体实施方式
[0026]
以下通过实施例对本发明的上述内容做进一步详细说明,但不应该将此理解为本发明上述主题的范围仅限于以下的实施例,凡基于本发明上述内容实现的技术均属于本发明的范围。
[0027]
实施例1
[0028][0029]
向15ml反应管中,依次加入化合物1a、有机溶剂、催化剂、添加剂及化合物2a,在空气条件下将反应管密封,将其置于加热模块中升温搅拌反应。待反应结束后,冷却至室温,加水淬灭反应,乙酸乙酯萃取,有机相干燥后,抽滤,旋干,硅胶柱分离(石油醚/乙酸乙酯=10/1)得白色固体产物3a。
[0030]
通过改变反应的溶剂、催化剂、银盐添加剂、醋酸盐添加剂、反应温度以及物料比等反应条件,结果见表1。
[0031]
表1不同条件下3a的合成
a
[0032]
[0033][0034]
实施例2
[0035][0036]
向15ml耐压管中,依次加入1a(63.5mg,0.36mmol)、[rhcp*cl2]2(4.6mg,0.0075 mmol)、agsbf6(10.3mg,0.03mmol)、zn(oac)2(16.5mg,0.09mmol)、乙酸乙酯(1.5 ml)和2a(48.9mg,0.3mmol),将反应管密封并置于110℃反应模块中搅拌反应10h。反应结束后,将反应体系冷却至室温,加水淬灭反应,乙酸乙酯萃取,有机相干燥后,抽滤,旋干,硅胶柱分离(石油醚/乙酸乙酯=10/1)得白色固体产物3a(69.3mg,83%)。1h nmr(400mhz,dmso
‑
d6):1h nmr(cdcl3,400mhz):δ7.82(dd,j1=8.4hz,j2= 1.2hz,2h),7.77(d,j=8.0hz,1h),7.73
‑
156.5,136.0,134.9,132.9,130.9,129.3,120.0,111.3,97.0,55.6,35.4,29.8.hrms(esi)m/z:[m h]
calcdforc
19
h
21
n2o2309.1598;found309.1599.
[0046]
(2
‑
(tert
‑
butyl)
‑6‑
isopropyl
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(phenyl)methanone(3d)
[0047]1hnmr(cdcl3,600mhz):δ7.83(d,j1=8.4hz,j2=1.2,2h),7.71
‑
7.68(m,1h),7.67(d,j=8.4hz,1h),7.52(t,j=8.4hz,2h),7.10(dd,j1=8.4hz,j2=1.2,1h),6.35(d,j=1.2hz,1h),2.79
‑
2.75(m,1h),1.56(s,9h),1.05(d,j=6.6hz,6h).
13
c{1h}nmr(cdcl3,150mhz):δ170.9,162.9,144.5,139.8,135.6,134.8,133.2,130.9,129.2,122.1,119.3,109.8,35.4,34.2,29.8,24.2.hrms(esi)m/z:[m h]
calcdforc
21
h
25
n2o321.1961;found321.1967.
[0048]
(2
‑
(tert
‑
butyl)
‑6‑
fluoro
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(phenyl)methanone(3e)
[0049]1hnmr(cdcl3,600mhz):δ7.81(dd,j1=8.4hz,j2=1.2hz,2h),7.75
‑
7.72(m,1h),7.69
‑
7.67(m,1h),7.56
‑
7.53(m,2h),6.98
‑
6.95(m,1h),6.25(dd,j1=9.0hz,j2=2.4hz,1h),1.56(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ170.3,163.8(d,4j
c
‑
f
=3.6hz),159.5(d,1j
c
‑
f
=239.8hz),137.8,135.4(d,3j
c
‑
f
=13.0hz),135.2,132.4,130.9,129.5,120.4(d,3j
c
‑
f
=9.4hz),111.3(d,2j
c
‑
f
=24.5hz),99.3(d,2j
c
‑
f
=28.1hz),35.5,29.7.
19
fnmr(cdcl3,565mhz):δ
‑
117.60
‑‑
117.64(m).hrms(esi)m/z:[m h]
calcdforc
18
h
18
fn2o297.1398;found297.1401.
[0050]
(2
‑
(tert
‑
butyl)
‑6‑
chloro
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(phenyl)methanone(3f)
[0051]1hnmr(cdcl3,600mhz):δ7.82
‑
7.80(m,2h),7.75
‑
7.72(m,1h),7.67(d,j=8.4hz,1h),7.56
‑
7.53(m,2h),7.20(dd,j1=9.0hz,j2=2.4hz,1h),6,57(d,j=1.8hz,1h),1.55(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ170.2,164.0,140.1,135.9,135.4,132.3,130.9,129.5,128.9,123.8,120.5,112.1,35.5,29.7.hrms(esi)m/z:[m h]
calcdforc
18
h
18
cln2o313.1102;found313.1104.
[0052]
(6
‑
bromo
‑2‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(phenyl)methanone(3g)
[0053]1hnmr(cdcl3,400mhz):δ7.82
‑
7.80(m,2h),7.74(t,j=7.6hz,1h),7.62(d,j=8.4hz,1h),7.55(t,j=8.0hz,2h),7.34(dd,j1=8.8hz,j2=2.0hz,1h),6.73(d,j=1.6hz,1h),1.55(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ170.2,163.8,140.5,136.4,135.4,132.3,131.0,129.5,126.5,121.0,116.4,114.9,35.5,29.7.hrms(esi)m/z:[m h]
calcdforc
18
h
18
brn2o357.0597;found357.0585.
[0054]
(2
‑
(tert
‑
butyl)
‑6‑
iodo
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(phenyl)methanone(3h)
[0055]1hnmr(cdcl3,600mhz):δ7.80(dd,j1=8.4hz,j2=1.2hz,2h),7.76
‑
7.73(m,1h),7.57
‑
7.52(m,4h),6.92(s,1h),1.54(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ170.2,163.6,141.0,136.8,135.4,132.3,132.2,131.0,129.5,121.4,120.8,86.7,35.4,29.7.hrms(esi)m/z:[m h]
calcdforc
18
h
18
in2o405.0458;found405.0454.
[0056]
(2
‑
(tert
‑
butyl)
‑6‑
nitro
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(phenyl)methanone
hz,j2=1.2hz,1h),1.57(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ170.3,163.7,138.9,136.4,135.2,132.5,131.0,129.4,124.7,123.7,123.0,110.6,35.6,29.7.hrms(esi)m/z:[m h]
calcdforc
18
h
18
cln2o313.1102;found313.1106.
[0068]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(p
‑
tolyl)methanone(3o)
[0069]1hnmr(cdcl3,600mhz):δ7.76(d,j=7.8hz,1h),7.72(d,j=7.8hz,2h),7.31(d,j=7.8hz,2h),7.22(t,j=7.8hz,1h),7.05
‑
7.02(m,1h),6.62(d,j=8.4hz,1h),2.47(s,3h),1.56(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ170.6.163.1,146.4,141.4,135.5,131.1,130.2,130.0,123.2,123.0,119.7,112.1,35.4,29.8,21.9.hrms(esi)m/z:[m h]
calcdforc
19
h
21
n2o293.1648;found293.1643.
[0070]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(4
‑
methoxyphenyl)methanone(3p)
[0071]1hnmr(cdcl3,600mhz):δ7.80
‑
7.78(m,2h),7.77(d,j=8.4hz,1h),7.23
‑
7.20(m,1h),7.07
‑
7.04(m,1h),6.98
‑
6.95(m,2h),6.69(d,j=7.8hz,1h),3.90(s,3h),1.55(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ169.9,165.2,163.0,141.3,135.7,133.6,125.0,123.1,122.9,119.6,114.6,111.9,55.7,35.3,29.8.hrms(esi)m/z:[m h]
calcdforc
19
h
21
n2o2309.1598;found309.1590.
[0072]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(4
‑
(tert
‑
butyl)phenyl)methanone(3q)
[0073]1hnmr(cdcl3,600mhz):δ7.78
‑
7.74(m,3h),7.52
‑
7.50(m,2h),7.23
‑
7.21(m,1h),7.06
‑
7.03(m,1h),6.64(d,j=7.8hz,1h),1,56(s,9h),1.37(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ170.6,163.1,159.3,141.4,135.6,131.0,130.0,126.3,123.2,122.9,119.6,112.1,35.5,35.4,31.0,29.8.hrms(esi)m/z:[m h]
calcdforc
22
h
27
n2o335.2118;found335.2118.
[0074]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(4
‑
fluorophenyl)methanone(3r)
[0075]1hnmr(cdcl3,400mhz):δ7.88
‑
7.84(m,2h),7.77(d,j=8.0hz,1h),7.25
‑
7.17(m,3h),7.08
‑
7.03(m,1h),6.59(d,j=8.0hz,1h),1,56(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ169.5,166.8(d,1j
c
‑
f
=257.1hz),163.1,141.5,135.3,133.8(d,3j
c
‑
f
=9.3hz),129.2(d,4j
c
‑
f
=2.8hz),123.4,123.2,119.9,116.7(d,2j
c
‑
f
=22.4hz),111.9,35.4,29.7.
19
fnmr(cdcl3,565mhz):δ
‑
101.0
‑‑
101.1(m).hrms(esi)m/z:[m h]
calcdforc
18
h
18
fn2o297.1398;found297.1398.
[0076]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(4
‑
chlorophenyl)methanone(3s)
[0077]1hnmr(cdcl3,600mhz):δ7.77
‑
7.75(m,3h),7.51
‑
7.48(m,2h),7.25
‑
7.22(m,1h),7.07
‑
7.04(m,1h),6.58(d,j=7.8hz,1h),1.56(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ169.7,163.2,141.7,141.4,135.2,132.3,131.2,129.8,123.5,123.3,119.9,112.0,35.4,29.7.hrms(esi)m/z:[m h]
calcdforc
18
h
18
cln2o313.1102;found313.1091.
[0078]
(4
‑
bromophenyl)(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)methanone(3t)
[0079]1hnmr(cdcl3,400mhz):δ7.77(d,j=8.0hz,1h),7.70
‑
7.65(m,4h),7.26
‑
7.22(m,1h),7.08
‑
7.04(m,1h),6.58(d,j=8.0hz,1h),1.56(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ169.9,163.2,141.4,135.2,132.8,132.3,131.7,130.5,123.5,123.3,119.9,112.0,35.4,29.7.hrms(esi)m/z:[m h]
calcdforc
18
h
18
brn2o357.0597;found357.0592.
[0080]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(4
‑
iodophenyl)methanone(3u)
[0081]1hnmr(cdcl3,400mhz):δ7.89(d,j=8.4hz,2h),7.77(d,j=8.0hz,1h),7.52(d,j=8.4hz,2h),7.24(t,j=7.6hz,1h),7.06(t,j=7.6hz,1h),6.58(d,j=8.4hz,1h),1.55(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ170.2,163.2,141.4,138.8,135.2,132.3,132.0,123.5,123.3,119.9,112.0,103.6,35.4,29.7.hrms(esi)m/z:[m h]
calcdforc
18
h
18
in2o405.0458;found405.0451.
[0082]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(4
‑
(trifluoromethyl)phenyl)methanone(3v)
[0083]1hnmr(cdcl3,400mhz):δ7.99(d,j=8.0hz,2h),7.85
‑
7.81(m,3h),7.31
‑
7.27(m,1h),7.11
‑
7.07(m,1h),6.53(d,j=8.0hz,1h),1.62(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ169.6,163.3,141.5,136.08,136.05(q,2j
c
‑
f
=32.9hz),135.0,131.2,126.4(q,3j
c
‑
f
=3.3hz),123.6,123.5,123.3(q,1j
c
‑
f
=271.2hz),120.0,112.1,35.5,29.7.
19
fnmr(cdcl3,565mhz):δ
‑
63,27(s).hrms(esi)m/z:[m h]
calcdforc
19
h
18
f3n2o347.1366;found347.1368.
[0084]
[1,1'
‑
biphenyl]
‑4‑
yl(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)methanone(3w)
[0085]1hnmr(cdcl3,600mhz):δ7.89(dt,j1=9.0hz,j2=2.4hz,2h),7.78(d,j=8.4hz,1h),7.73
‑
7.72(m,2h),7.65
‑
7.64(m,2h),7.49
‑
7.47(m,2h),7.44
‑
7.41(m,1h),7.24
‑
7.22(m,1h),7.07
‑
7.04(m,1h),6.68(d,j=7.8hz,1h),1.59(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ170.5,163.2,147.8,141.5,139.2,135.5,131.6,131.4,129.2,128.8,127.9,127.4,123.3,123.1,119.7,112.2,35.5,29.8.hrms(esi)m/z:[m h]
calcdforc
24
h
23
n2o355.1805;found355.1806.
[0086]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(m
‑
tolyl)methanone(3x)
[0087]1hnmr(cdcl3,400mhz):δ7.76(d,j=8.0hz,1h),7.68(s,1h),7.55(d,j=7.6hz,1h),7.49(d,j=8.0hz,1h),7.36(t,j=7.6hz,1h),7.22
‑
7.19(m,1h),7.04
‑
7.00(m,1h),6.58(d,j=8.4hz,1h),2.40(s,3h),1.57(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ170.9,163.3,141.4,139.4,135.8,135.5,133.0,131.2,129.1,128.2,123.2,123.0,119.7,112.2,35.4,29.8,21.3.hrms(esi)m/z:[m h]
calcdforc
19
h
21
n2o293.1648;found293.1646.
[0088]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(3
‑
chlorophenyl)methanone(3y)
[0089]1hnmr(cdcl3,400mhz):δ7.87(t,j=1.6hz,1h),7.77(d,j=8.0hz,1h),7.69
‑
7.66(m,1h),7.65
‑
7.62(m,1h),7.45(tj=8.0hz,1h),7.26
‑
7.22(m,1h),7.08
‑
7.04(m,1h),6.56(d,j=8.0hz,1h),1.57(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ169.5,163.3,
141.5,135.7,135.1,134.9,134.7,130.58,130.57,128.9,123.5,123.4,119.9,112.1,35.5,29.7.hrms(esi)m/z:[m h]
calcdforc
18
h
18
cln2o313.1102;found313.1100.
[0090]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(o
‑
tolyl)methanone(3z)
[0091]1hnmr(cdcl3,600mhz):δ7.73(d,j=8.4hz,1h),7.54
‑
7.51(m,1h),7.41(d,j=7.8hz,1h),7.37(dd,j1=7.8hz,j2=0.6hz,1h),7.24(t,j=7.8hz,1h),7.21
‑
7.18(m,1h),6.98
‑
6.95(m,1h),6.27(d,j=8.4hz,1h),2.54(s,3h),1.63(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ170.2,163.5,141.6,140.0,134.8,133.30,133.25,132.2,130.8,126.6,123.5,123.3,119.8,112.3,35.8,29.7,20.4.hrms(esi)m/z:[m h]
calcdforc
19
h
21
n2o293.1648;found293.1646.
[0092]
(2
‑
bromophenyl)(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)methanone(3aa)
[0093]1hnmr(cdcl3,400mhz):δ7.74
‑
7.71(m,2h),7.57
‑
7.54(m,1h),7.50
‑
7.45(m,2h),7.24
‑
7.19(m,1h),6.99
‑
6.95(m,1h),6.18(d,j=8.4hz,1h),1.66(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ167.6,163.4,141.8,136.0,134.6,134.3,133.7,131.3,128.2,123.9,123.8,121.4,120.1,112.4,36.0,29.4.hrms(esi)m/z:[m h]
calcdforc
18
h
18
brn2o357.0597;found357.0606.
[0094]
benzo[d][1,3]dioxol
‑4‑
yl(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)methanone(3bb)
[0095]1hnmr(cdcl3,600mhz):δ7.77(d,j=8.4hz,1h),7.37(dd,j1=8.4hz,j2=1.8hz,1h),7.32(d,j=1.8hz,1h),7.24
‑
7.22(m,1h),7.09
‑
7.07(m,1h),6.86(d,j=8.4hz,1h),6.74(d,j=8.4hz,1h),6.11(s,2h),1.55(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ169.6,162.9,153.7,148.7,141.3,135.6,128.2,126.8,123.2,123.0,119.7,111.9,110.2,108.7,102.5,35.4,29.7.hrms(esi)m/z:[m h]
calcdforc
19
h
19
n2o3323.1390;found323.1394.
[0096]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(naphthalen
‑1‑
yl)methanone(3cc)
[0097]1hnmr(cdcl3,400mhz):δ8.64(d,j=8.8hz,1h),8.15(d,j=8.4hz,1h),8.00(d,j=7.6hz,1h),7.75(d,j=8.0hz,1h),7.70
‑
7.65(m,3h),7.45(t,j=7.6hz,1h),7.19
‑
7.15(m,1h),6.89
‑
6.85(m,1h),6.27(d,j=8.0hz,1h),1.67(s,9h).
13
c{1h}nmr(cdcl3,100mhz):δ169.9,163.7,141.5,135.2,134.9,134.2,131.2,131.1,130.3,128.99,128.96,127.2,125.1,124.8,123.4,123.3,119.8,112.6,35.8,29.7.hrms(esi)m/z:[m h]
calcdforc
22
h
21
n2o329.1648;found329.1649.
[0098]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(thiophen
‑2‑
yl)methanone(3dd)
[0099]1hnmr(cdcl3,600mhz):δ7.86(dd,j1=4.8hz,j2=1.2hz,1h),7.77(d,j=8.4hz,1h),7.53(dd,j1=3.6hz,j2=1.2hz,1h),7.24(td,j1=7.8hz,j2=1.2hz,1h),7.15
‑
7.14(m,1h),7.13
‑
7.10(m,1h),6.93(d,j=8.4hz,1h),1.55(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ164.2,162.4,141.4,137.7,137.4,137.1,135.8,128.9,123.3,123.1,119.7,111.6,35.4,29.7.hrms(esi)m/z:[m h]
calcdforc
16
h
17
n2os285.1056;
found285.1046.
[0100]
(2
‑
(tert
‑
butyl)
‑4‑
methyl
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(furan
‑2‑
yl)methanone(3ee)
[0101]1hnmr(cdcl3,600mhz):δ7.71(dd,j1=1.8hz,j2=0.6hz,1h),7.21(dd,j1=3.6hz,j2=0.6hz,1h),7.05
‑
7.00(m,2h),6.66(d,j=7.8hz,1h),6.62(dd,j1=3.6hz,j2=1.8hz,1h),2.67(s,3h),1.53(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ161.4,159.6,149.0,147.6,140.8,135.3,130.0,123.5,123.2,123.1,113.3,108.7,35.4,29.7,16.7.hrms(esi)m/z:[m h]
calcdforc
17
h
19
n2o2283.1441;found283.1441.
[0102]1‑
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)ethan
‑1‑
one(3ff)
[0103]1hnmr(cdcl3,600mhz):δ8.02(d,j=7.8hz,1h),7.97(d,j=8.4hz,1h),7.81
‑
7.78(m,1h),7.54
‑
7.51(m,1h),2.91(s,3h),1.49(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ172.7,167.3,149.8,132.9,129.0,126.3,124.7,122.3,39.4,29.6,21.9.hrms(esi)m/z:[m na]
calcdforc
13
h
16
n2nao239.1155;found239.1159.
[0104]1‑
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)hexan
‑1‑
one(3gg)
[0105]1hnmr(cdcl3,600mhz):δ8.06(d,j=8.4hz,1h),7.98(d,j=8.4hz,1h),7.80
‑
7.77(m,1h),7.53
‑
7.50(m,1h),3.24(t,j=7.8hz,2h),1.94
‑
1.89(m,2h),1.51(s,9h),1.46
‑
1.39(m,4h),0.93(t,j=7.2hz,3h).
13
c{1h}nmr(cdcl3,150mhz):δ172.7,170.4,150.1,132.6,129.1,126.1,124.3,121.8,39.5,34.2,31.7,29.6,28.0,22.6,14.1.hrms(esi)m/z:[m h]
calcdforc
17
h
25
n2o273.1961;found273.1959.
[0106]1‑
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)
‑3‑
cyclohexylpropan
‑1‑
one(3hh)
[0107]1hnmr(cdcl3,600mhz):δ8.05(d,j=8.4hz,1h),7.97(d,j=8.4hz,1h),7.79
‑
7.77(m,1h),7.52(t,j=7.8hz,1h),3.25(t,j=7.8hz,2h),1.85(d,j=13.2hz,2h),1.79
‑
1.71(m,4h),1.67
‑
1.65(m,1h),1.49(s,9h),1.38
‑
1.33(m,1h),1.27
‑
1.20(m,3h),1.02
‑
0.96(m,2h).
13
c{1h}nmr(cdcl3,150mhz):δ172.7,170.8,150.1,132.6,129.1,126.2,124.3,121.7,39.5,37.5,36.0,33.3,31.9,29.6,26.7,26.4.hrms(esi)m/z:[m h]
calcdforc
20
h
29
n2o313.2274;found313.2270.
[0108]1‑
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)
‑4‑
methylpentan
‑1‑
one(3ii)
[0109]1hnmr(cdcl3,600mhz):δ8.05(d,j=8.4hz,1h),7.97(d,j=8.4hz,1h),7.80
‑
7.77(m,1h),7.52(t,j=7.2hz,1h),3.25(t,j=7.8hz,2h),1.78(q,j=7.8hz,2h),1.73
‑
1.69(m,1h),1.49(s,9h),1.00(d,j=6.6hz,6h).
13
c{1h}nmr(cdcl3,150mhz):δ172.7,170.7,150.1,132.6,129.1,126.2,124.3,121.7,39.5,37.4,29.7,29.6,28.0,22.5.hrms(esi)m/z:[m h]
calcdforc
17
h
25
n2o273.1961;found273.1960.
[0110]1‑
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)
‑3‑
phenylpropan
‑1‑
one(3jj)
[0111]1hnmr(cdcl3,400mhz):δ8.03(d,j=7.6hz,1h),7.98(d,j=8.4hz,1h),7.81
‑
7.77(m,1h),7.53
‑
7.49(m,1h),7.31
‑
7.27(m,4h),7.22
‑
7.18(m,1h),3.58(t,j=8.4hz,2h),3.28(t,j=8.4hz,2h),1.50(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ172.7,169.0,150.1,141.7,132.7,129.2,128.5,128.4,126.3,126.1,124.1,121.8,39.6,35.8,33.7,29.6.hrms(esi)m/z:[m h]
calcdforc
20
h
23
n2o307.1805;found307.1821.
[0112]1‑
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)
‑3‑
(p
‑
tolyl)propan
‑1‑
one(3kk)
[0113]1hnmr(cdcl3,600mhz):δ8.01(d,j=8.4hz,1h),7.97(d,j=8.4hz,1h),7.77(t,j=7.8hz,1h),7.49(t,j=7.8hz,1h),7.17(d,j=7.2hz,2h),7.09(d,j=7.8hz,2h),3.54(t,j=7.8hz,2h),3.22(t,j=8.4hz,2h),2.31(s,3h),1.50(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ172.7,169.2,150.1,138.7,135.5,132.7,129.1,128.4,126.3,124.1,121.8,39.6,36.1,33.3,29.6,21.0.hrms(esi)m/z:[m h]
calcdforc
21
h
25
n2o321.1961;found321.1954.
[0114]1‑
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)
‑3‑
(4
‑
chlorophenyl)propan
‑1‑
one(3ll)
[0115]1hnmr(cdcl3,600mhz):δ8.00
‑
7.97(m,2h),7.80
‑
7.77(m,1h),7.52
‑
7.50(m,1h),7.23(d,j=8.4hz,2h),7.18(d,j=8.4hz,2h),3.54(t,j=8.4hz,2h),3.25(t,j=7.8hz,2h),1.48(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ172.6,168.6,150.1,140.1,132.8,131.8,129.9,129.2,128.5,126.4,123.9,121.7,39.6,35.5,32.8,29.6.hrms(esi)m/z:[m h]
calcdforc
20
h
22
cln2o341.1415;found341.1410.
[0116]
(2
‑
(tert
‑
butyl)
‑
1h
‑
benzo[d]imidazol
‑1‑
yl)(cyclohexyl)methanone(3mm)
[0117]1hnmr(cdcl3,400mhz):δ7.77
‑
7.75(m,1h),7.39
‑
7.36(m,1h),7.32
‑
7.27(m,2h),3.33
‑
3.25(m,1h),2.07(d,j=13.6hz,2h),1.91
‑
1.87(m,2h),1.78
‑
1.62(m,3h),1.54(s,9h),1.41
‑
1.30(m,3h).
13
c{1h}nmr(cdcl3,100mhz):δ179.1,162.8,141.8,133.7,123.8,123.5,120.3,111.7,47.3,35.8,29.7,29.6,25.55,25.50.hrms(esi)m/z:[m h]
calcdforc
18
h
25
n2o285.1961;found285.1951.
[0118]
实施例4
[0119]
本发明所合成产物n
‑
酰基苯并咪唑类化合物3进行一系列反应,从而合成进一步的衍生物。例如:
[0120][0121]
向15ml耐压管中依次加入3a(55.7mg,0.2mmol)和5ml的四氢呋喃,在0℃下加入lialh4(15.2mg,0.4mmol),在空气氛围下将反应管密封,升至室温,并在室温条件下反应2h。反应结束后,加入饱和氯化铵溶液淬灭反应,然后将反应混合物转移至分液漏斗中,乙酸乙酯(10ml
×
3)萃取。合并有机相,无水硫酸钠干燥,有机相干燥后,抽滤,旋干,硅胶柱分离(石油醚/乙酸乙酯=3/1)得白色固体产物4(30.7mg,88%)。1hnmr(dmso
‑
d6,600mhz):δ12.1(s,1h),7.52
‑
7.41(m,2h),7.11
‑
7.10(m,2h),1.40(s,9h).
13
c{1h}nmr(dmso,150mhz):δ162.6,143.3,135.1,121.9,121.2,118.8,111.2,33.6,29.7.hrms(esi)m/z:[m h]
calcdforc
11
h
15
n2175.1230;found175.1230.
[0122]
实施例5
[0123]
本发明所合成产物n
‑
酰基苯并咪唑类化合物3进行一系列反应,从而合成进一步
的衍生物。例如:
[0124][0125]
向15ml耐压管中依次加入3aa(35.7mg,0.1mmol)、dmso(1ml)、pd(oac)2(1.1 mg,0.005mmol)、pph3(5.2mg,0.02mmol)、k3po4(25.5mg,0.12mmol)和苯乙炔(16.5μl, 0.15mmol),氩气条件下将反应管密封,放置在80℃的模块中反应24h。反应结束后,加入水稀释,然后将反应混合物转移至分液漏斗中,乙酸乙酯(10ml
×
3)萃取。合并有机相,无水硫酸钠干燥,有机相干燥后,抽滤,旋干,硅胶柱分离(石油醚/乙酸乙酯=20/1)得白色固体产物5(32.9mg,87%)。1h nmr(cdcl3,400mhz):δ7.88(d,j =7.6hz,1h),7.78(d,j=8.0hz,1h),7.69
‑
7.66(m,2h),7.61
‑
7.56(m,1h),7.33
‑
7.30(m, 3h),7.26
‑
7.22(m,3h),7.05
‑
7.01(m,1h),6.42(d,j=8.4hz,1h),1.64(s,9h).
13
c{1h} nmr(cdcl3,100mhz):δ169.0,163.0,141.8,135.6,134.9,134.3,133.0,131.9,130.8, 128.92,128.89,128.3,123.7,123.54,123.47,121.9,119.8,112.1,95.2,85.6,35.7,29.6. hrms(esi)m/z:[m h]
calcd for c
26
h
23
n2o 379.1805;found 379.1800.
[0126]
实施例6
[0127]
本发明所合成产物n
‑
酰基苯并咪唑类化合物3进行一系列反应,从而合成进一步的衍生物。例如:
[0128][0129]
向15ml耐压管中依次加入3aa(71.5mg,0.2mmol)、哌啶(1ml)、pd(pph3)4(4.6mg, 0.004mmol)和cui(3.8mg,0.02mmol),在空气条件下将反应管密封,室温条件下反应 15h。反应结束后,加入饱和氯化铵溶液淬灭,然后将反应混合物转移至分液漏斗中,乙酸乙酯(10ml
×
3)萃取。合并有机相,无水硫酸钠干燥,有机相干燥后,抽滤,旋干,硅胶柱分离(石油醚/乙酸乙酯=10/1)得白色固体产物6(23.2mg,32%)。1h nmr (cdcl3,600mhz):δ7.72(d,j=7.8hz,1h),7.52(t,j=7.2hz,1h),7.47(d,j=7.2hz, 1h),7.18(t,j=7.8hz,1h),7.09(d,j=8.4hz,1h),7.02(t,j=7.8hz,1h),6.93(t,j= 7.8hz,1h),6.32(d,j=9.0hz,1h),2.97(br s,2h),2.79(br s,2h),1.65(s,9h),1.41
‑
1.37 (m,2h),1.30
‑
1.26(m,4h).
13
c{1h}nmr(cdcl3,150mhz):δ168.6,163.6,153.7,141.7, 135.0,134.1,132.3,126.6,123.3,123.2,121.6,119.9,119.7,113.0,53.8,36.3,29.3,25.9, 23.8.hrms(esi)m/z:[m h]
calcd for c
23
h
27
n3nao 384.2046;found 384.2068.
[0130]
实施例7
[0131]
本发明所合成产物n
‑
酰基苯并咪唑类化合物3进行一系列反应,从而合成进一步的衍生物。例如:
[0132][0133]
向15ml耐压管中依次加入3aa(35.7mg,0.1mmol)、1,4
‑
二氧六环(1ml)、pd(oac)2(2.2mg,0.01mmol)、pph3(15.7mg,0.06mmol)、k2co3(55.3mg,0.4mmol)和苯硼酸(13.4mg,0.11mmol),氩气条件下将反应管密封,放置于80℃模块中反应24h。反应结束后,加水稀释,然后将反应混合物转移至分液漏斗中,乙酸乙酯(10ml
×
3)萃取。合并有机相,无水硫酸钠干燥,有机相干燥后,抽滤,旋干,硅胶柱分离(石油醚/乙酸乙酯=10/1)得白色固体产物7(32.6mg,92%)。1hnmr(cdcl3,600mhz):δ7.69
‑
7.65(m,2h),7.57(dd,j1=7.8hz,j2=1.8hz,1h),7.50
‑
7.45(m,2h),7.26
‑
7.16(m,6h),7.00
‑
6.98(m,1h),6.30(d,j=8.4hz,1h),1.54(s,9h).
13
c{1h}nmr(cdcl3,150mhz):δ169.1,163.8,142.9,141.7,139.1,134.5,134.0,132.6,131.7,130.0,128.5,128.3,128.0,127.8,123.6,123.5,119.9,113.3,36.2,29.3.hrms(esi)m/z:[m h]
calcdforc
24
h
23
n2o355.1805;found355.1804.
[0134]
以上实施例描述了本发明的基本原理、主要特征及优点。本行业的技术人员应该了解,本发明不受上述实施例的限制,上述实施例和说明书中描述的只是说明本发明的原理,在不脱离本发明原理的范围下,本发明还会有各种变化和改进,这些变化和改进均落入本发明保护的范围内。
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。