1.本发明属于生物医药领域,具体涉及顺式烯酰胺类衍生物在制备抗菌药物中的应用。
背景技术:
2.黄皮(clausena lansium(lour.))的各个部位长期以来一直是传统的民间药材。从黄皮果核提取出来的一种黄皮酰胺属化合物——lansiumamide b,由于其多样的结构和生物活性,得到医药和农药化学家们的广泛研究,据报道lansiumamide b具有抗肥胖和提高胰岛素敏感性、抗惊厥和解痉挛、抗炎和抗坏死活性等药理活性。并且lansiumamide b具有显著的杀虫、抑菌和除草农用活性。
[0003][0004]
lansiumamide b是从黄皮植株提取出来的一种黄皮酰胺属化合物。黄皮(clausena lansium(lour.))是一种绿灌木或小乔木,芸香科(rutaceae)家族的成员,别名油梅、鸡皮果。黄皮(clausena lansium)起源于中国大陆南部,因其广泛的生物活性而在中国大陆南部、东南亚和北美洲广泛栽培。黄皮的各个部位长期以来一直是传统的民间药材,素有“饥食荔枝,饱食黄皮”的说法。民间喜欢拿水煎黄皮叶来防治感冒、支气管哮喘、胃肠道炎症和脓肿,或者用黄皮树根来治气痛。黄皮的果皮和果核也都是入药良材,有利尿消肿、行气止痛等功效。黄皮种子富含油分,出油率高达42%,为优良的润滑剂。黄皮作为一种优质水果以酸甜芳香的味道在顾客中广受欢迎,其果实除鲜食外,可以制成水果杯、明胶等甜点。
技术实现要素:
[0005]
本发明的目的是提供顺式烯酰胺类衍生物在制备抗菌药物中的应用。
[0006]
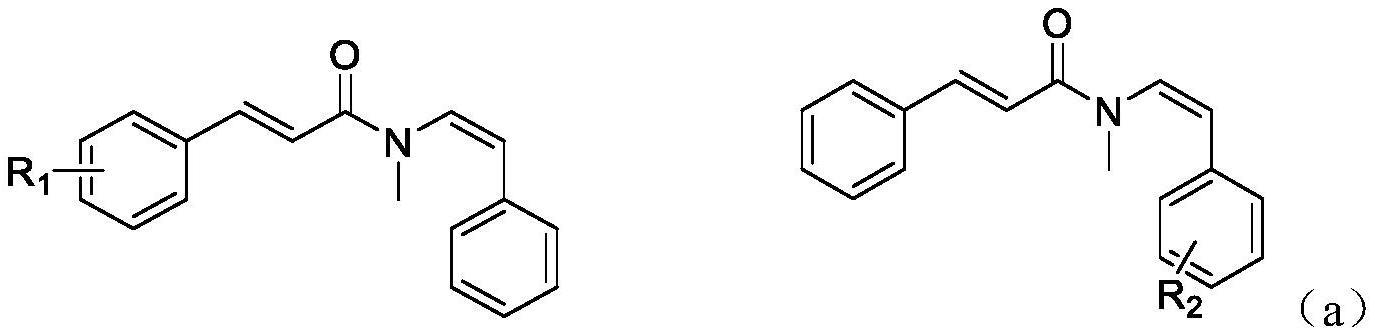
所述的顺式烯酰胺类衍生物,其化学结构如(a)所示,
[0007][0008]
(a)式中,r1是氢,甲氧基,甲基,三氟甲基,苯并稠环或卤素等;
[0009]
r2是甲基,甲氧基,三氟甲基,卤素等。
[0010]
本发明所述的顺式烯酰胺类衍生物优选下述化合物之一:
[0011][0012]
进一步优选,所述的顺式烯酰胺类衍生物的结构式如下任一所示:
[0013][0014][0015]
本发明通过实验发现,顺式烯酰胺类衍生物能有效抑制植物致病真菌,具有良好的抑菌活性,可用于治疗植物致病真菌感染的农药。
[0016]
优选,是上述顺式烯酰胺类衍生物或其盐在制备植物源抑菌农药中的应用。
[0017]
优选,是顺式烯酰胺类衍生物或其盐在制备抗核盘菌、水稻纹枯或灰霉菌中的应用。
[0018]
本发明所述的顺式烯酰胺类衍生物具有抑菌作用,这为农业上植物致病真菌提供一种新结构类型的农药。
具体实施方式
[0019]
以下实施例是对本发明的进一步说明,而不是对本发明的限制。
[0020]
化合物2002-2046的结构式如下式所示:
[0021][0022]
实施例1化合物2002-2018的合成。
[0023][0024]
化合物1的合成:
[0025]
250ml的两口烧瓶、磁子、抽气头提前放烘箱干燥,安装好烧瓶,抽真空至室温换入氩气,迅速称入碘仿(21mmol)、三苯基膦(22mmol),再次抽真空换入氩气三次,将长针头吸取的无水thf(80ml)打入烧瓶。加入t-buok(20mmol),1min后,加入芳香醛衍生物(10mmol)的无水thf(15ml)溶液。常温搅拌30min,将悬浮液冷却至-78℃后,分批加入t-buok(50mmol)。15分钟后,滴入饱和食盐水淬灭,让溶液慢慢恢复至室温。乙酸乙酯(3x 50ml)萃取,有机相合并,然后用无水的硫酸钠干燥,最后减压蒸发溶剂。得到粗液用石油醚洗脱液,硅胶层析柱纯化。得到化合物1。
[0026]
化合物2的合成:
[0027]
称取化合物1(9.8mmol)于100ml的封管中,加入磁子,量入thf(20ml)和等量的水,称入4-甲基苯磺酰肼(19.6mmol)和乙酸钠(29.4mmol),旋紧封管塞,于油浴下加热回流反应。用tlc监测反应,待起始原料已完全消耗(12h)后,撤去油浴,将反应混合物冷却至室温,冰浴下加入饱和氯化铵水溶液淬灭反应,然后用et2o(3
×
20ml)萃取,用饱和氯化钠水溶液洗涤有机相,用无水的硫酸钠干燥。滤出有机相,在减压下除去溶剂,残余物过柱子。用石油醚为洗脱剂,纯化得到化合物2。
[0028]
化合物3的合成:
[0029]
schlenk管称入cui(0.25mmol,5mol%)、cs2co3(7.5mmol)和反式肉桂酰胺(0.882g,6.0mmol),抽真空换入氩气。加入dmeda (0.50mmol,10mol%)、化合物2(5.0mmol)和thf(10.0ml)。把schlenk管密封,置于60℃油浴下反应。在tlc分析表明起始原料已完全消耗(3h)后,撤去油浴换为冰浴降温,待恢复到室温。用硅藻土滤除固体,乙酸乙酯洗涤(50ml)。用真空旋转蒸发仪除去溶剂后将得到粗品。用石油醚/乙酸乙酯(v/v=5:1)为洗脱剂,过柱子纯化后得到化合物3。
[0030]
化合物4的合成:
[0031]
0℃下,向含有化合物3(4.13mmol)的无水dmf(15ml)中加入60%nah(24.78mmol)。反应30min后,滴入碘甲烷(28.91mmol)。在tlc分析表明起始原料已完全消耗(2h)后,缓慢滴入水淬灭反应。乙酸乙酯(3
×
10ml)萃取有机物,有机相合并用饱和食盐水洗涤,然后用
无水naso4干燥,最后减压蒸发溶剂。得到粗液用石油醚/乙酸乙酯(v/v=10:1)为洗脱液,硅胶层析柱纯化得到目标化合物2002-2018。
[0032]
2002:yellowish oil.1h nmr(400mhz,cdcl3)δ:7.48(d,j=15.6hz,1h),7.45-7.43(m,2h),7.38-7.32(m,3h),7.22(dd,j=7.6hz,1h),7.18(td,j=7.6,1.6hz,1h),6.91(d,j=15.6hz,1h),6.88(t,j=7.6hz,1h),6.76(d,j=7.6hz,1h),6.50(d,j=8.8hz,1h),6.41(d,j=8.8hz,1h),3.75(s,3h),3.06(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.5,156.7,142.0,135.4,129.6,129.3,129.2,129.1,128.7(2
×
c),128.0(2
×
c),123.6,120.7,119.7,118.8,110.5,55.4,34.9.hrms(esi)m/z calculated for c
19h20
o2n
[m h]
294.1489,found,294.1493.
[0033]
2003:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.62(d,j=15.6 hz,1h),7.47-7.44(m,2h),7.36-7.32(m,3h),7.21(t,j=8.0 hz,1h),6.92(d,j=15.6 hz,1h),6.91(d,j=8.0 hz,1h),6.85(s,1h),6.78(dd,j=8.0,2.4 hz,1h),6.49(d,j=8.8 hz,1h),6.21(d,j=8.8 hz,1h),3.74(s,3h),3.10(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.5,159.7,142.7,135.8,135.2,129.8,129.7,129.1,128.8(2
×
c),128.0(2
×
c),124.9,121.2,118.4,113.9,113.8,55.3,34.8.hrms(esi)m/z calculated for c
19h20
o2n
[m h]
294.1489,found 294.1493.
[0034]
2004:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.65(d,j=15.6 hz,1h),7.46-7.43(m,2h),7.35-7.31(m,3h),7.28(d,j=8.8 hz,2h),6.95(d,j=15.6 hz,1h),6.83(d,j=8.8 hz,2h),6.37(d,j=8.4 hz,1h),6.20(d,j=8.4 hz,1h),3.77(s,3h),3.10(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.6,159.4,142.6,135.3,130.2(2
×
c),129.7,128.8(2
×
c),128.0(2
×
c),127.0,126.9,125.5,118.4,114.1(2
×
c),55.3,34.5.hrms(esi)m/z calculated for c
19h20
o2n
[m h]
294.1489,found 294.1499.
[0035]
2005:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.59(d,j=15.6 hz,1h),7.51-7.49(m,2h),7.38-7.35(m,3h),7.23-7.20(m,1h),7.16-7.13(m,3h),6.92(d,j=15.6 hz,1h),6.65(d,j=8.8 hz,1h),6.22(d,j=8.8 hz,1h),2.94(s,3h),2.28(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.6,142.7,136.1,135.2,134.1,130.2,129.8,129.5,128.8(2
×
c),128.6,128.0(2
×
c),127.9,126.1,121.2,118.4,34.9,20.1.hrms(esi)m/z calculated for c
19h20
on
[m h]
278.1539,found 278.1544.
[0036]
2006:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.62(d,j=15.6 hz,1h),7.48-7.45(m,2h),7.37-7.33(m,3h),7.20(t,j=7.6 hz,1h),7.13(d,j=7.6 hz,1h),7.11(s,1h),7.04(d,j=7.6 hz,1h),6.93(d,j=15.6 hz,1h),6.48(d,j=8.8 hz,1h),6.20(d,j=8.8 hz,1h),3.09(s,3h),2.29(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.6,142.6,138.3,135.3,134.5,129.8,129.5,128.9,128.8(2
×
c),128.7,128.6,128.0(2
×
c),125.8,125.0,118.5,34.8,21.5.hrms(esi)m/z calculated for c
19h20
on
[m h]
278.1539,found 278.1545.
[0037]
2007:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.65(d,j=15.6 hz,1h),7.47-7.44(m,2h),7.36-7.32(m,3h),7.23(d,j=8.0 hz,2h),7.11(d,j=8.0 hz,2h),6.95(d,j=15.6 hz,1h),6.44(d,j=8.8 hz,1h),6.22(d,j=8.8 hz,1h),3.10(s,3h),2.31(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.5,142.6,138.2,135.2,131.5,129.7,129.4(2
×
c),
128.8(2
×
c),128.6(2
×
c),128.1,128.0(2
×
c),125.5,118.3,24.6,21.3.hrms(esi)m/z calculated for c
19h20
on
[m h]
278.1539,found 278.1548.
[0038]
2008:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.57(d,j=15.6 hz,1h),7.48-7.45(m,2h),7.39-7.33(m,3h),7.30(d,j=7.6 hz,1h),7.20(dd,j=13.2,6.4 hz,1h),7.06(t,j=7.6 hz,1h),6.98(t,j=9.2 hz,1h),6.90(d,j=15.6 hz,1h),6.62(d,j=8.8 hz,1h),6.32(d,j=8.8 hz,1h),3.07(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.4,160.1(d,j=248 hz),142.9,135.1,130.9,129.9,129.8(d,j=8.9 hz),129.7,128.8(2
×
c),128.0(2
×
c),124.3(d,j=3.0 hz),122.6(d,j=13.9 hz),118.2,116.2(d,j=3.7 hz),115.7(d,j=21.7 hz),34.8.
19
f nmr(376 mhz,cdcl3)δ-114.9.hrms(esi)m/z calculated for c
18h17
onf
[m h]
282.1289,found 282.1296.
[0039]
2009:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.64(d,j=15.6 hz,1h),7.48-7.45(m,2h),7.36-7.33(m,3h),7.27(dd,j=14.0,8.0 hz,1h),7.10(d,j=8.0 hz,1h),7.00(d,j=10.0hz,1h),6.93(td,j=8.0,2.4 hz,1h),6.90(d,j=15.6 hz,1h),6.56(d,j=8.8 hz,1h),6.19(d,j=8.8 hz,1h),3.09(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.6,162.9(d,j=245 hz),143.2(2
×
c),136.7(d,j=7.8 hz),135.1,130.3(d,j=8.3 hz),130.0(d,j=16 hz),128.9(2
×
c),128.1(2
×
c),124.5(d,j=2.3 hz),123.4,118.1,115.4(d,j=21.8 hz),115.1(d,j=21.1 hz),34.9.
19
f nmr(376 mhz,cdcl3)δ-112.6.hrms(esi)m/z calculated for c
18h17
onf
[m h]
282.1289,found 282.1297.
[0040]
2010:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.63(d,j=15.6 hz,1h),7.46-7.43(m,2h),7.35-7.27(m,5h),6.98(t,j=8.8 hz,2h),6.91(d,j=15.6 hz,1h),6.47(d,j=8.8 hz,1h),6.20(d,j=8.8 hz,1h),3.08(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.5,162.2(d,j=247hz),142.9,135.1,130.6(d,j=3.3 hz),130.5(d,j=8.0 hz,2
×
c),129.9,128.8(2
×
c),128.7,128.0(2
×
c),124.1,118.2,115.8(d,j=21.5 hz,2
×
c),34.6.
19
f nmr(376 mhz,cdcl3)δ-112.5.hrms(esi)m/z calculated for c
18h17
onf
[m h]
282.1289,found 282.1297.
[0041]
2011:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.54(d,j=15.6 hz,1h),7.50-7.47(m,2h),7.38-7.34(m,3h),7.30-7.27(m,2h),7.21-7.12(m,2h),6.88(d,j=15.6 hz,1h),6.64(d,j=8.8 hz,1h),6.37(d,j=8.8 hz,1h),3.02(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.4,142.9,135.1,133.5,133.3,130.7,129.9,129.7,129.1,128.8(2
×
c),128.1(3
×
c),127.0,119.9,118.1,35.1.hrms(esi)m/z calculated for c
18h17
oncl
[m h]
298.0993,found 298.0998.
[0042]
2012:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.67(d,j=15.6 hz,1h),7.53-7.48(m,2h),7.42-7.36(m,3h),7.29(s,1h),7.25-7.21(m,3h),6.92(d,j=15.6 hz,1h),6.59(d,j=8.8hz,1h),6.17(d,j=8.8 hz,1h),3.10(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.5,143.1,136.4,135.0,134.5,130.2,129.9(2
×
c),128.8(2
×
c),128.7(2
×
c),128.0(2
×
c),126.6,122.7,118.0,34.9.hrms(esi)m/z calculated for c
18h17
clno
[m h]
298.0993,found 298.1000.
[0043]
2013:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.66(d,j=15.6 hz,1h),7.49-7.46(m,2h),7.38-7.35(m,3h),7.28(abq,j=8.8 hz,4h),6.92(d,j=15.6 hz,1h),6.55
(d,j=8.8 hz,1h),6.21(d,j=8.8 hz,1h),3.10(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.5,143.1,135.1,133.8,133.0,130.0(2
×
c),129.9,129.5,129.0(2
×
c),128.8(2
×
c),128.1(2
×
c),123.7,118.1,34.8.hrms(esi)m/z calculated for c
18h17
oncl
[m h]
298.0993,found 298.0999.
[0044]
2014:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.58(d,j=7.2 hz,1h),7.57(d,j=15.6hz,1h),7.50-7.45(m,3h),7.39-7.29(m,5h),6.88(d,j=15.6 hz,1h),6.69(d,j=8.8 hz,1h),6.43(d,j=8.8 hz,1h),2.96(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.6,143.3,134.9,133.6,132.0,131.1,130.3,130.0,128.8(2
×
c),128.1(2
×
c),128.0(q,j=29.6 hz),127.8,126.1(q,j=5.4 hz),124.2(q,j=272 hz)118.5,117.9,35.1.
19
f nmr(376 mhz,cdcl3)δ-60.7.hrms(esi)m/z calculated for c
19h17
onf
3
[m h]
332.1257,found 332.1265.
[0045]
2015:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.62(d,j=15.6 hz,1h),7.53-7.49(m,2h),7.48-7.40(m,4h),7.35-7.32(m,3h),6.88(d,j=15.6 hz,1h),6.61(d,j=8.8 hz,1h),6.22(d,j=8.8 hz,1h),3.07(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.5,143.2,135.5,135.0,131.5,131.1(q,j=32.1 hz),130.6,130.0,129.2,128.8(2
×
c),128.0(2
×
c),125.6(q,j=3.7 hz),124.6,123.9(q,j=271 hz),122.4,118.0,34.9.
19
f nmr(376 mhz,cdcl3)δ-62.9.hrms(esi)m/z calculated for c
19h17
onf
3
[m h]
332.1257,found 332.1263.
[0046]
2016:yellowish oil.1h nmr(400mhz,cdcl3)δ:7.61(d,j=15.6hz,1h),7.54(d,j=8.0hz,2h),7.47-7.43(m,2h),7.39(d,j=8.0hz,2h),7.37-7.33(m,3h),6.88(d,j=15.6hz,1h),6.63(d,j=8.8hz,1h),6.23(d,j=8.8hz,1h),3.08(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.5,143.3,138.3,135.0,131.0,130.0,129.8(q,j=33hz),128.9(2
×
c),128.8(2
×
c),128.0(2
×
c),125.6(q,j=3.3hz,2
×
c),124.0(q,j=265hz),122.7,118.0,35.0.
19
f nmr(376mhz,cdcl3)δ-62.7;hrms(esi)m/z calculated for c
19h17
onf
3
[m h]
332.1257,found 332.1265.
[0047]
2017:yellowish oil.1h nmr(400mhz,cdcl3)δ:8.00(dd,j=8.0hz,1h),7.82(d,j=8.0hz,1h),7.76(t,j=4.4hz,1h),7.58(d,j=15.6hz,1h),7.55-7.46(m,3h),7.45-7.41(m,2h),7.38-7.35(m,2h),7.34-7.30(m,2h),6.95(d,j=15.6hz,1h),6.86(d,j=8.8hz,1h),6.67(d,j=8.8hz,1h),2.90(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.6,142.9,135.1,133.7,132.1,131.4,130.7,129.8,128.8(2
×
c),128.4,128.0(2
×
c),126.7,126.5,126.1(2
×
c),125.6,124.2,119.4,118.3,34.9.hrms(esi)m/z calculated for c
22h20
on
[m h]
314.1539,found 314.1544.
[0048]
2018:yellowish oil.1h nmr(400mhz,cdcl3)δ:7.80-7.75(m,4h),7.66(d,j=15.6hz,1h),7.49-7.43(m,5h),7.33-7.31(m,3h),7.00(d,j=15.6hz,1h),6.60(d,j=8.8hz,1h),6.39(d,j=8.8hz,1h),3.13(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.8,143.0,135.2,133.3,132.9,132.1,129.8,129.2,128.8(2
×
c),128.5,128.4,128.2,128.0(2
×
c),127.7,126.6,126.5,126.1,124.7,118.4,35.0.hrms(esi)m/z calculated for c
22h20
on
[m h]
314.1539,found314.1546.
[0049]
实施例2化合物2021-2046的合成。
[0050][0051]
化合物a的合成:
[0052]
100ml两口烧瓶、抽气头和磁子烘箱烘干,取出后组装抽真空至室温换入氩气,称取邻甲氧基苯甲醛(1.36g,10mmol)于样品瓶中,吸取无水thf(40ml)打入烧瓶,称取乙氧甲酰基亚甲基三苯基膦(4.176g,12mmol),冰浴条件下缓慢加入烧瓶,冰浴反应10min后撤去冰浴,反应隔夜后,冰浴条件下,加入饱和氯化铵溶液(20ml)淬灭反应,减压蒸发除去thf,加入乙酸乙酯(20ml)萃取,饱和氯化钠溶液(20ml)洗涤1次,有机相使用无水硫酸钠干燥30min,减压蒸发除去溶剂,柱层析纯化,洗脱剂为石油醚和乙酸乙酯(v:v=20:1),得到无色油状液体a(2.02g,9.8mmol),产率:98%。
[0053]
化合物b的合成:
[0054]
取10ml封管,称入a(206mg,1mmol),加入氨-甲醇溶液(2ml),旋紧旋塞,室温反应3天,减压蒸发除去溶剂得粗品,柱层析纯化,洗脱剂为石油醚和丙酮(v:v=5:1)得到白色固体b(124mg,0.70mol),产率76%。
[0055]
化合物c的合成:
[0056]
取100ml封管,加入磁子,称取碘(2.80g,11.0mmol),冲入氩气(持续1min),反扣橡胶塞,插入粗针头,吸取无水苯(12ml)打入封管,加入无水吗啡啉(2.62ml,30.0mmol),室温反应30min。称取苯乙炔(2.25g,10.0mmol)于样品瓶中,加入无水苯(10ml)稀释缓慢滴入封管中,再用无水苯(8ml)分两次洗涤样品瓶打入封管。油浴加热至45℃反应24h,自然冷却至室温后使用硅藻土过滤,乙酸乙酯洗涤(3
×
20ml)。收集滤液,饱和氯化铵溶液(20ml)、饱和氯化钠溶液(20ml)依次洗涤1次,收集有机相无水硫酸钠干燥30min,旋转蒸发除去溶剂,柱层析纯化,洗脱剂为石油醚,得到黄色油状液体c(2.23g,9.8mmol),产率:98%。
[0057]
化合物d的合成:
[0058]
取100ml的封管,称取c(2.23g,9.8mmol)于封管中,加入磁子、thf(20ml)和水(20ml),称取4-甲基苯磺酰肼(3.65g,19.6mmol)、无水乙酸钠(2.41g,29.4mmol)加入封管,
旋紧封管塞,120℃油浴反应12h后,自然冷却至室温,冰浴下加入饱和氯化铵溶液淬灭反应,乙酸乙酯(3
×
20ml)萃取,饱和氯化钠溶液(20ml)洗涤1次,有机相无水硫酸钠干燥30min,旋转蒸发除去溶剂,柱层析纯化,洗脱剂为石油醚,得到无色油状液体d(1.31g,5.68mmol),产率:58%。
[0059]
化合物e的合成:
[0060]
取10ml schlenk管和磁子烘干,组装后(塞上橡胶塞)抽真空至室温换入氩气,加入b(195mg,1.1mmol),称入cui(9.5mg,0.05mmol,)、cs2co3(407mg,1.25mmol),称取d(230mg,1mmol),吸取无水thf(5ml),2.5ml稀释d1,2.5ml分两次洗涤样品瓶打入。再滴入dmeda(10.8μl,0.1mmol),换橡胶塞为涂少量油脂的玻璃塞,密封schlenk管,65℃油浴下反应。反应3h后,撤去油浴,自然恢复到室温。硅藻土滤除固体,乙酸乙酯洗涤(30ml)。旋转蒸发除去溶剂。柱层析纯化,洗脱剂为石油醚和乙酸乙酯(v/v=5:1),纯化后得到淡黄色固体e(209mg,0.75mmol),产率:75%。
[0061]
化合物s的合成:
[0062]
取25ml两口烧瓶、磁子和抽气头烘干,组装装置抽真空至室温换入氩气,称取e(0.75mmol),吸取无水dmf(3ml),冰浴条件下,加入纯度为60%的nah(4.5mmol)。保持冰浴反应30min后,缓慢滴入碘甲烷(5.25mmol)。室温反应3h,冰浴条件下,缓慢滴入水淬灭反应。乙酸乙酯(3
×
10ml)萃取,合并有机相,饱和氯化钠溶液洗涤1次,有机相无水硫酸钠干燥30min,旋转蒸发除去溶剂。柱层析纯化,洗脱液为石油醚和乙酸乙酯(v/v=10:1),得到目标化合物s。
[0063]
2021:yellowish oil.1h nmr(400mhz,cdcl3)δ:7.59(d,j=15.6hz,1h),7.33-7.27(m,4h),7.25-7.20(m,2h),7.05(d,j=7.6hz,1h),6.96(s,1h),6.91(d,j=15.6hz,1h),6.88(dd,j=7.6,2.0hz,1h),6.49(d,j=8.8hz,1h),6.24(d,j=8.8hz,1h),3.81(s,3h),3.09(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.4,159.8,142.6,136.6,134.5,129.8,128.9,128.7(4
×
c),128.2,125.2,120.6,118.7,115.4,113.2,55.4,34.7.hrms(esi)m/z calculated for c
19h20
o2n
[m h]
294.1489,found 294.1492.
[0064]
2023:yellowish oil.1h nmr(400mhz,cdcl3)δ:7.90(d,j=15.6hz,1h),7.40(d,j=7.6hz,1h),7.34-7.28(m,4h),7.26-7.20(m,2h),7.17-7.13(m,2h),6.84(d,j=15.6hz,1h),6.49(d,j=8.8hz,1h),6.23(d,j=8.8hz,1h),3.10(s,3h),2.38(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.6,140.6,137.7,134.5,134.3,130.7,129.5,128.9,128.7(4
×
c),128.2,126.3,126.2,125.2,119.6,34.7,19.9.hrms(esi)m/z calculated for c
19h20
on
[m h]
278.1539,found278.1542.
[0065]
2024:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.64(d,j=15.6 hz,1h),7.37-7.31(m,4h),7.30-7.23(m,4h),7.17(d,j=7.2 hz,1h),6.94(d,j=15.6 hz,1h),6.54(d,j=8.8 hz,1h),6.27(d,j=8.8 hz,1h),3.11(s,3h),2.37(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.7,143.1,138.5,135.2,134.6,130.7,129.0,128.9(2
×
c),128.8(2
×
c),128.7,128.6,128.2,125.3,125.0,118.1,34.8,21.5.hrms(esi)m/z calculated for c
19h20
on
[m h]
278.1539,found278.1542.
[0066]
2027:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.55(d,j=15.6 hz,1h),7.32-7.27(m,5h),7.24-7.22(m,1h),7.19(d,j=8.0 hz,1h),7.11(d,j=10.0 hz,1h),7.01
(td,j=8.0,2.0 hz,1h),6.91(d,j=15.6 hz,1h),6.46(d,j=8.8 hz,1h),6.27(d,j=8.8 hz,1h),3.10(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.0,163.0(d,j=245 hz),141.2(d,j=2.6 hz),137.5(d,j=7.7 hz),134.3,130.3(d,j=8.2 hz),128.8(2
×
c),128.7(3
×
c),128.2,125.6,124.1(d,j=2.7hz),119.7,116.6(d,j=21.3 hz),114.0(d,j=21.6 hz),34.7.
19
f nmr(376 mhz,cdcl3)δ:-113.0.hrms(esi)m/z calculated for c
18h17
onf
[m h]
282.1289,found 282.1292.
[0067]
2028:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.57(d,j=15.6 hz,1h),7.42(dd,j=8.8,5.6 hz,2h),7.32-7.27(m,4h),7.25-7.20(m,1h),7.02(t,j=8.8 hz,2h),6.84(d,j=15.6hz,1h),6.48(d,j=8.8 hz,1h),6.25(d,j=8.8 hz,1h),3.09(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.4,163.6(d,j=249 hz),141.5,134.5,131.5(d,j=3.3 hz),129.8(d,j=8.3 hz,2
×
c),128.9(d,j=7.6 hz,2
×
c),128.8(2
×
c),128.7(2
×
c),128.2,125.3,118.1(d,j=1.9 hz),115.9(d,j=21.6 hz),34.8.
19
f nmr(376 mhz,cdcl3)δ:-110.6.hrms(esi)m/z calculated for c
18h17
onf
[m h]
282.1289,found 282.1292.
[0068]
2033:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.64(s,1h),7.60(d,j=15.6 hz,1h),7.58-7.56(m,2h),7.45(t,j=7.6 hz,1h),7.34-7.28(m,4h),7.25-7.20(m,1h),6.98(d,j=15.6hz,1h),6.48(d,j=8.8 hz,1h),6.31(d,j=8.8 hz,1h),3.14(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:165.7,140.7,136.0,134.3,131.2,131.1(q,j=32.2 hz),129.3,128.8(2
×
c),128.6(3
×
c),128.2,126.0(q,j=3.5 hz),125.7,124.2(q,j=3.6 hz),123.9(q,j=271 hz),120.3,34.7.
19
f nmr(376 mhz,cdcl3)δ:-62.8.hrms(esi)m/z calculated for c
19h17
onf
3
[m h]
332.1257,found 332.1260.
[0069]
2036:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.86-7.77(m,5h),7.59(dd,j=8.4,1.2hz,1h),7.52-7.46(m,2h),7.36-7.29(m,4h),7.25-7.20(m,1h),7.05(d,j=15.6 hz,1h),6.55(d,j=8.8 hz,1h),6.28(d,j=8.8 hz,1h),3.12(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.6,142.8,134.5,134.1,133.4,132.7,129.7,129.0,128.8(4
×
c),128.6,128.5,128.2,127.8,127.0,126.7,125.1,123.8,118.5,34.8.hrms(esi)m/z calculated for c
22h20
on
[m h]
314.1539,found 314.1543.
[0070]
2037:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.96(d,j=15.6 hz,1h),7.42(d,j=7.6hz,1h),7.25-7.28(m,5h),7.25-7.21(m,1h),7.03(d,j=15.6 hz,1h),6.91(t,j=7.6 hz,1h),6.88(d,j=7.6 hz,1h),6.52(d,j=8.8 hz,1h),6.20(d,j=8.8 hz,1h),3.84(s,3h),3.07(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:167.1,158.3,138.3,134.7,131.0,129.2,128.9,128.8(2
×
c),128.7(2
×
c),128.0,124.3,124.2,120.6,119.0,111.1,55.5,34.7.hrms(esi)m/z calculated for c
19h20
o2n
[m h]
294.1489,found 294.1492.
[0071]
2038:yellowish oil.1h nmr(400 mhz,cd3od)δ:7.41(d,j=15.6 hz,1h),7.39(d,j=8.8 hz,2h),7.29-7.27(m,4h),7.22-7.17(m,1h),6.88(d,j=8.8 hz,2h),6.85(d,j=15.6 hz,1h),6.52(d,j=8.8 hz,1h),6.38(d,j=8.8 hz,1h),3.8(s,3h),3.06(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:168.7,162.7,143.6,135.9,130.6(2
×
c),129.8,129.7(2
×
c),129.6(2
×
c),129.1,128.8,127.3,116.6,115.2(2
×
c),55.8,35.0.hrms(esi)m/z calculated for c
19h20
o2n
[m h]
294.1489,found,294.1492.
[0072]
2039:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.63(d,j=15.6 hz,1h),7.36(d,j=8.0hz,2h),7.34-7.28(m,4h),7.25-7.20(m,1h),7.15(d,j=8.0 hz,2h),6.90(d,j=15.6 hz,1h),6.51(d,j=8.8 hz,1h),6.23(d,j=8.8 hz,1h),3.08(s,3h),2.35(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.7,142.8,140.1,134.5,132.5,129.5(2
×
c),129.0,128.8(2
×
c),128.7(2
×
c),128.1,128.0(2
×
c),124.9,117.2,34.7,21.5.hrms(esi)m/z calculated for c
19h20
on
[m h]
278.1539,found 278.1542.
[0073]
2040:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.70(d,j=15.6 hz,1h),7.42(td,j=7.6,1.6 hz,1h),7.32-7.27(m,5h),7.25-7.20(m,1h),7.10(t,j=7.6 hz,1h),7.06(dd,j=7.6,1.6hz,1h),7.04(d,j=15.6 hz,1h),6.48(d,j=8.8 hz,1h),6.25(d,j=8.8 hz,1h),3.10(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.4,161.4(d,j=252 hz),135.6,134.5,131.1(d,j=8.6hz),129.6(d,j=3.2 hz),128.8,128.7(4
×
c),128.2,125.3,124.3(d,j=3.5 hz),123.3(d,j=11.6 hz),121.2(d,j=7.3 hz),116.2(d,j=21.8 hz),34.8.
19
f nmr(376 mhz,cdcl3)δ:-114.1.hrms(esi)m/z calculated for c
18h17
onf
[m h]
282.1289,found 282.1292.
[0074]
2041:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.98(d,j=15.6 hz,1h),7.43(dd,j=7.6,2.0 hz,1h),7.36(dd,j=7.6,1.6 hz,1h),7.33-7.30(m,4h),7.26-7.18(m,3h),6.91(d,j=15.6hz,1h),6.47(d,j=8.8 hz,1h),6.24(d,j=8.8 hz,1h),3.10(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.0,138.7,134.9,134.4,133.6,130.5,130.1,128.8(3
×
c),128.7(2
×
c),128.2,127.7,126.9,125.4,121.2,34.8.hrms(esi)m/z calculated for c
18h17
oncl
[m h]
298.0993,found298.0996.
[0075]
2042:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.52(d,j=15.6 hz,1h),7.39(s,1h),7.32-7.20(m,8h),6.91(d,j=15.6 hz,1h),6.47(d,j=8.8 hz,1h),6.27(d,j=8.8 hz,1h),3.10(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.0,141.1,137.1,134.7,134.3,130.0,129.6,128.8(2
×
c),128.7(3
×
c),128.3,127.5,126.4,125.7,119.8,34.8.hrms(esi)m/z calculated for c
18h17
oncl
[m h]
298.0993,found 298.0996.
[0076]
2043:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.54(d,j=15.6 hz,1h),7.36(d,j=8.4hz,2h),7.32-7.27(m,6h),7.24-7.20(m,1h),6.89(d,j=15.6 hz,1h),6.47(d,j=8.8 hz,1h),6.25(d,j=8.8 hz,1h),3.09(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:166.2,141.3,135.6,134.4,133.7,129.2(2
×
c),129.1(2
×
c),128.9(2
×
c),128.8,128.7(2
×
c),128.3,125.5,118.9,34.8.hrms(esi)m/z calculated for c
18h17
oncl
[m h]
298.0993,found 298.0996.
[0077]
2044:yellowish oil.1h nmr(400 mhz,cdcl3)δ:7.97(dq,j=15.6,2.0 hz,1h),7.65(d,j=7.6 hz,1h),7.50-7.37(m,3h),7.36-7.29(m,4h),7.27-7.21(m,1h),6.86(d,j=15.6 hz,1h),6.45(d,j=8.8 hz,1h),6.25(d,j=8.8 hz,1h),3.11(s,3h).
13
c{1h}nmr(100 mhz,cdcl3)δ:165.6,138.4,134.5,134.3,131.9,130.1,129.1,128.8(2
×
c),128.7(2
×
c),128.6,128.4,128.0,126.1(q,j=5.6hz),125.6,124.0(q,j=272hz),122.9,34.8.
19
f nmr(376mhz,cdcl3)δ:-62.8.hrms(esi)m/z calculated for c
19h17
onf
3
[m h]
332.1257,found 332.1260.
[0078]
2045:yellowish oil.1h nmr(400mhz,cdcl3)δ:7.48(d,j=15.6hz,1h),7.44
(abq,j=8.4hz,4h),7.21-7.18(m,4h),7.15-7.10(m,1h),6.88(d,j=15.6hz,1h),6.36(d,j=8.8hz,1h),6.19(d,j=8.8hz,1h),3.02(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:165.8,140.8,138.7,134.3,131.2(q,j=32.3hz),128.9(2
×
c),128.7(2
×
c),128.6,128.3,128.1(2
×
c),125.9,125.8(q,j=3.7hz,2
×
c),124.0(q,j=270hz),121.0,34.8.
19
f nmr:(376mhz,cdcl3)δ-62.7.hrms(esi)m/z calculated for c
19h17
onf
3
[m h]
332.1257,found 332.1260.
[0079]
2046:yellowish oil.1h nmr(400mhz,cdcl3)δ:8.46(d,j=15.2hz,1h),8.15(d,j=7.6hz,1h),7.93-7.85(m,2h),7.59-7.51(m,3h),7.45(t,j=7.6hz,1h),7.39-7.32(m,4h),7.28-7.24(m,1h),7.02(d,j=15.2hz,1h),6.53(d,j=8.8hz,1h),6.28(d,j=8.8hz,1h),3.18(s,3h).
13
c{1h}nmr(100mhz,cdcl3)δ:166.4,140.0,134.5,133.7,132.9,131.6,130.0,128.9,128.8(2
×
c),128.7(2
×
c),128.6,128.4,126.7,126.2,125.5,125.4,124.8,123.9,121.3,34.8.hrms(esi)m/z calculated for c
22h20
on
[m h]
314.1539,found 314.1543.
[0080]
实施例2抑菌实验
[0081]
对本发明的对顺式烯酰胺类衍生物进行了抑菌试验。
[0082]
马铃薯培养基的制备(pda)
[0083]
马铃薯葡萄糖水25g,琼脂20g,蒸馏水1000ml。称取马铃薯葡萄糖水25g,称取琼脂20g,加热溶解于1000ml双蒸水中,分装于锥形瓶,设置温度121℃时间15分钟以上高压灭菌后置于阴暗处备用。
[0084]
病原真菌的保存与培养
[0085]
灭菌后的pda培养基用微波炉加热成流动态,于超净工作台上将15ml的培养基倒入直径为9cm的无菌培养皿中,凝固后接入0.5cm的菌饼,密封后28℃下培养,待菌株长满整个培养皿后,再置于5℃的冰箱中保存,每两个月活化一次即可。
[0086]
抑菌实验
[0087]
以核盘菌为例子:
[0088]
a)将保存在5℃冰箱的核盘菌,提前24小时转放到28℃恒温生化培养箱下备用。
[0089]
b)在无菌条件下,将用dmso溶解供试药品,抽滤除菌,配制成母液浓度5.00
[0090]
mg/ml。
[0091]
c)用手动移液器分别吸取0.3ml、0.15ml、0ml母液到2ml离心管中,补dmso至0.3ml,补水至1ml。倒入49ml的pda培养基中(培养基提前冷却至55℃上下),两者充分摇匀后趁热倒入培养皿(提前灭过菌),每皿10ml,冷却,备用,此培养基中最终化合物浓度为50μg/ml、25μg/ml、0μg/ml,dmso最终含量为1%。
[0092]
d)准备预先活化纯化过的供试菌种,使用5mm直径打孔器制备菌饼,打孔位置选培养皿边缘。用接种环将菌饼有菌丝面朝下转移至上述准备好的带药培养基正中央,每皿放1个菌饼。每一浓度重复做3组,做好标记后在28℃恒温培养箱中培养,倒置存放。
[0093]
e)培养24h后(真菌不同,菌丝的生长速率不同,观察的时间不同,选取空白处理组菌饼直径长到培养皿70-80%,来观察药液处理组的情况),菌落直径十字交叉法测量,记录并采用下列供试计算菌丝生长抑制率:
[0094]
菌落扩展直径(mm)=菌落直径平均值-5(菌饼直径,mm)
[0095]
菌丝生长抑制率(%)=(对照菌落扩展直径-处理菌落扩展直径)/对照菌落扩展直径x 100%
[0096]
f)根据观测结果,设定5组浓度梯度,重复c-e步骤,计算相应抑菌率。
[0097]
g)数据用excel 2004处理计算抑菌率及抑菌率标准方差,拟合毒力回归方程,用spss
[0098]
24软件,通过“p”分析,计算半数有效浓度(51%effective concentration,ec
50
)、95%置信区间。并采用下列供试计算不同化合物对lansiumamide b的相对毒力系数:
[0099]
相对毒力系数=lansiumamide b对真菌的ec
50
/目标化合物对真菌的ec
50
[0100]
化合物对3株病原真菌的抑制活性
[0101]
(1)初步评价lansiumamide b及其衍生物对3株病原真菌的抑制活性
[0102]
以市售多菌灵为阳性对照,测定了在50μg/ml,25μg/ml浓度下合成的34个化合物对核盘菌、水稻纹枯菌和灰霉菌的抑制率。从表1可以看出lansiumamide b及其衍生物其对三种病原真菌,在50μg/ml的药液浓度下均没有达到99%的抑制率,与多菌灵活力差距大。lansiumamide b及其衍生物中,50μg/ml的药液浓度下,对核盘菌的抑制率在58.03~97.83%范围内,除2004外,25μg/ml的药液浓度下,对核盘菌的抑制率也多数在34%以上;lansiumamide b及其衍生物中,50μg/ml的药液浓度下,对水稻纹枯的抑制率在56.72~95.44%,除2017外,25μg/ml的药液浓度下,对核盘菌的抑制率也多数在38%以上;lansiumamide b及其衍生物对灰霉菌抑菌效果不明显,50μg/ml的药液浓度下,抑制率最高是2009,仅达到51.3%。
[0103]
表1衍生物对3株病原真菌抑制率(%)
[0104]
[0105][0106]
(2)部分lansiumamide b衍生物对2株病原真菌的抑制活性
[0107]
选择在初筛浓度下对核盘菌抑制效果明显优于lansiumamide b的6个化合物进行毒力测定,并以市售多菌灵为阳性对照。结果如表2所示,2008、2011、2028、2037对核盘菌抑制活性较lansiumamide b有显著提高,相对毒力是2.00~/10.78倍,其中2037的抑菌效果最好,是lansiumamide b的10.78倍,其ec
50
仅有1.51μg/ml。2002、2005对核盘菌的ec
50
与lansiumamide b的无差异显著。
[0108]
表3-5衍生物对核盘菌的抑制效果
[0109][0110]
又选了6个化合物在初筛浓度下对水稻纹枯抑制效果明显优于lansiumamide b的化合物进一步的测定毒力,以市售多菌灵为阳性对照药剂。结果如表3所示,2028对水稻纹枯菌抑制活性较lansiumamide b有显著提高,相对毒力是2.49倍,其ec
50
仅有2.04μg/ml。2002、2005、2008、2011和2040对水稻纹枯的抑菌效果均较lansiumamide b无显著差异。
[0111]
表3衍生物对水稻纹枯菌的抑制率
[0112]
再多了解一些
本文用于创业者技术爱好者查询,仅供学习研究,如用于商业用途,请联系技术所有人。