1.本发明属于化学合成领域,涉及一种手性环丁醇化合物的合成方法及该手性环丁醇化合物及用途。
背景技术:
2.具有手性中心的环丁烷结构广泛存在于药物分子和天然产物中。对映选择性[2 2]环加成反应是一类重要的构建手性环丁烷骨架的方法,toste在2011年报道了过渡金属金催化分子内的联烯与烯烃的不对称[2 2]环加成反应,反应底物需要有联烯存在(j.am.chem.soc.2011,133,5500.)。brown报道了利用手性路易斯酸催化联烯酸酯与简单烯烃的不对称分子间
[0003]
[2 2]环加成反应,类似的联烯底物是必要的,并且简单的烯烃底物仅有单取代烯烃或者环状烯烃,因此底物存在局限性(j.am.chem.soc.2015,137,3482.)。bach在2009年报道了利用手性光敏剂在光照条件下将能量传递给底物,但是这类方法需要底物与光敏剂通过氢键形式结合,也因此底物需要特定的官能团(angew.chem.int.ed.2009,48,6640)。基于此,为获得高对映选择性的简单的环丁烷结构分子目前现有的方法都存在一些局限性。环丁酮的制备简单易操作,通过手性还原试剂或者催化剂还原环丁酮获得手性环丁醇的方法暂时没有报道,只有toshio honda在1993年首次利用不对称还原策略将环丁烯酮还原成手性环丁烯醇中间体并应用在具有手性环丁烷骨架的单萜grandisol的合成(tetrahedron:asymmetry 1993,4,1537.)。通过醇导向实现醇γ位甲基的碳氢键功能化已经在2012年被hartwig报道(nature 2012,483,70.),因此通过不对称还原易得的环丁酮底物得到高对映立体选择性的环丁醇底物,然后发生醇导向的碳氢键功能化反应便能实现多手性中心的环丁烷骨架,并且能够得到含有季碳手性中心的环丁烷骨架。手性环丁烷骨架结构是一类重要的合成砌块,可以转化为多种多样的分子结构。因此,进一步发展高效的方法合成手性环丁烷中间体具有重要的理论与现实意义。
技术实现要素:
[0004]
本发明的目的是提供一类手性环丁醇的合成方法。即首先通过手性cbs催化剂将环丁酮还原成手性环丁醇,然后在铱催化下利用醇导向策略实现醇γ位甲基碳氢键选择性硅化反应,直接构建含有季碳手性中心的环丁醇化合物。
[0005]
为了达到上述目的,本发明提供了构建手性中心的手性环丁醇2的方法,包括:在cbs催化剂和还原剂作用下,带有不同取代基的环丁酮1在发生不对称还原,生成具有手性中心的环丁醇化合物。
[0006]
本发明中,合成手性环丁醇的操作步骤如下:
[0007]
1)方法一:在惰性气体氛围下,加入有机溶剂,cbs溶液,和一定bh3·
sme2的溶液,加入底物环丁酮;其中,cbs如下:
[0008][0009]
优选地,带有取代基的环丁酮1与cbs催化剂的用量的摩尔比为1:(0.01~0.50),带有取代基的环丁酮1与bh3·
sme2的用量的摩尔比为1:(0.1~5.0)。
[0010]
方法二:加入有机溶剂和(-)-(ipc)2bcl溶液,在-20℃下加入底物环丁酮溶液,在-20℃下反应;。
[0011]
2)待步骤1)反应完全后,淬灭反应,浓缩,快速柱层析得具有手性的环丁醇。
[0012]
合成手性环丁醇的操作步骤如反应式(i)所示:
[0013][0014]
进行硅化反应,构建含有季碳手性中心的环丁醇化合物,步骤如下:
[0015]
3)在惰性气体氛围下,使用钌催化剂,使得手性环丁醇2与硅氢试剂在室温下反应得到环丁醇硅醚衍生物;
[0016]
4)环丁醇硅醚衍生物在铱催化剂、菲啰啉配体和氢受体的作用下在20~150℃反应2~48h;
[0017]
5)方法一:在-78℃下冷却并向步骤4)的反应物中加入一定量有机金属试剂反应,得到具有季碳手性中心的环丁醇衍生物3。
[0018]
方法二:或者向步骤4)的反应物中加入一定量的khco3,氟化盐,过氧化物,meoh,并在50℃下加热反应得到具有季碳手性中心的环丁醇衍生物4。优选地,氟化盐为kf,过氧化物为h2o2。
[0019]
手性环丁醇在铱催化剂硅氢试剂(反应式以二乙基硅烷为例),菲啰啉配体,氢受体存在下发生甲基c-h键活化。反应过程如反应式(ii)所示:
[0020][0021]
其中,r1为烃基,带有官能团的烃基,苯基,芳基或杂环基;r2为烃基,带有官能团的烃基,苯基,芳基或者杂环基。
[0022]
优选地,反应式(i)中,r1为末端取代或非取代的c1-c20烃基,苯基,芳基或者杂环基;反应式(ii)中,r2为末端取代或非取代的c1-c10烃基,苯基,芳基或者杂环基。
[0023]
进一步地,反应式(i)中,r1为末端取代或非取代的c1-c10烃基,苯基,芳基或者杂环基;反应式(ii)中,r2为末端取代或非取代的c1-c10烃基,苯基,芳基或者杂环基。
[0024]
进一步地,反应式(i)中,r1选自c1-c10直链烷基,c1-c10环烷基,末端带有官能团的c1-c10烷基,苯基,芳基或者杂环基;反应式(ii)中r2选自c1-c10直链烷基,c1-c10环烷基,末端带有官能团的c1-c10烷基,苯基,芳基或者杂环基;进一步优选地,反应式(i)中,r1选自甲基,乙基,正丙基,异丙基,正丁基,正戊基,正己基,正庚基,正辛基,苯乙基,4-氯丁基,3-甲基丁基,3-氰基丙基,烯丙基;反应式(ii)中r2选自甲基,乙基,正丙基,叔丁基,苯基,邻甲基苯基,间甲基苯基,对甲基苯基,间甲氧基苯基,对氯苯基,对溴苯基,对酯基苯基,2-萘基,3-噻吩基。
[0025]
优选地,手性环丁醇2与硅氢试剂用量的摩尔比为1∶(0.1~5),手性环丁醇2与钌催化剂的用量的摩尔比为1∶(0.0001~0.5)。
[0026]
优选地,手性环丁醇2与铱催化剂的用量的摩尔比为1∶(0.0001~0.5),手性环丁醇2与菲啰啉配体用量的摩尔比为1∶(0.05~1),优选地,手性环丁醇2与有机金属的摩尔比为1∶(1~5)。
[0027]
优选地,本发明所述的钌催化剂为三(三苯基膦)二氯化钌(ii)。优选三(三苯基膦)二氯化钌(ii)。
[0028]
优选地,本发明所述的铱催化剂为二(1,5-环辛二烯)二-m-甲氧基二铱(i),(1,5-环辛二烯)氯化铱(i)二聚体,二(环辛烯)氯化铱(i)二聚体中的任意一种或多种。优选地,为二(1,5-环辛二烯)二-m-甲氧基二铱(i),(1,5-环辛二烯)氯化铱(i)二聚体。
[0029]
优选地,本发明所述的菲啰啉配体为l1,l2,l3,l4中的任意一种或多种。进一步地,为l1。
[0030][0031]
优选地,本发明所述的氢受体为降冰片烯,环辛二烯,双环[2.2.1]-5-庚烯-2-甲酸酯中的任意一种或多种。优选地,为双环[2.2.1]-5-庚烯-2-甲酸叔丁酯和降冰片烯。
[0032]
优选地,本发明所述的有机金属试剂选自有机金属锂试剂,有机金属镁试剂中的任意一种或多种,所述的有机金属锂试剂的结构通式为r-li,所述的有机金属镁试剂的结构通式为r-mgx,其中,r为c1~c6的烃基、苯基或芳基,所述芳基是邻、间、对位有c1~c6烃基取代的苯基。进一步地,所述的有机金属锂试剂为苯基锂。
[0033]
本发明的创新点在于,本发明通过以简单易得的官能化环丁酮为起始原料,在钌催化剂,甲酸三乙胺共沸物5:2的作用下,首次实现了一步合成具有高光学活性的环丁醇化合物。并且通过手性环丁醇在铱催化下实现碳氢键活化,实现了季碳手性环丁醇合成。
[0034]
本发明的有益效果还包括:原料和试剂简单易得,制备方便;反应条件温和,操作简单;底物普适性广;官能团兼容性好;产物具有高对映选择性(90%ee~>99%ee);产物易分离纯化等。
具体实施方式
[0035]
下面将对本发明的技术方案进行清楚、完整地描述,显然,所描述的实施例是本发明一部分实施例,而不是全部的实施例。基于本发明中的实施例,本领域普通技术人员在没有做出创造性劳动前提下所获得的所有其他实施例,都属于本发明保护的范围。
[0036]
具体实施方式如下:
[0037]
方法一:将干燥的反应管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气,依次加入一定体积的有机溶剂四氢呋喃,一定体积的1n的cbs的四氢呋喃溶液,和一定体积2n的bh3·
sme2的四氢呋喃溶液,室温下在一定时间内将一定体积的底物环丁酮的四氢呋喃溶液滴加到上述体系中,滴加完毕后室温搅拌1.0小时;其中,所述有机溶剂四氢呋喃的用量为1.0-5.0ml/mmol是以官能化环丁酮的用量为基准。优选地,为2.0ml/mmol。所述一定体积的in的cbs的四氢呋喃溶液的用量为0.1-1.0ml/mmol是以官能化环丁酮的用量为基准。优选地,为0.1ml/mmol。所述一定体积的2n的bh3·
sme2的四氢呋喃溶液指官能化环丁酮底物的用量为基准,所述的用量为0.1-1.0ml/mmol。优选地,为0.3ml/mmol。
[0038]
方法二:将干燥的反应管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气,依次加入一定体积的有机溶剂四氢呋喃,一定体积的1.7n的(-)-(ipc)2bcl的庚烷溶液,和,在-20℃下在逐滴加入一定体积的底物环丁酮的四氢呋喃溶液,滴加完毕后-20℃搅拌1.0小时;其中,所述一定体积的四氢呋喃指以官能化环丁酮底物用量为基准,所述有机溶剂的用量为1.0-5.0ml/mmol。优选地,为0.1ml/mmol。所述一定体积的1.7n的(-)-(ipc)2bcl的庚烷溶液指官能化环丁酮底物的用量为基准,所述的用量为0.1-1.0ml/mmol。优选地,为0.7ml/mmol。
[0039]
2)待步骤1)反应完全后,加入一定体积的甲醇淬灭反应,浓缩,快速柱层析得具有手性的环丁醇化合物;其中,所述一定体积的甲醇指以官能化环丁酮底物用量为基准,所述有机溶剂的用量为1.0-5.0ml/mmol。优选地,为1.0ml/mmol。
[0040]
3)待步骤2)得到手性环丁醇后。向一个干燥的反应管中加入钌催化剂,连接真空泵,在氩气氛围下置换氩气,依次加入一定体积的手性环丁醇的四氢呋喃溶液,和一定体积的二乙基硅烷,然后室温反应12小时,真空1小时抽干多余的四氢呋喃和二乙基硅烷得到环丁醇硅醚衍生物。所述一定体积的四氢呋喃溶液指官能化环丁酮底物的用量为基准,所述的用量为1.0-5.0ml/mmol。
[0041]
4)待步骤3)真空除去低沸点物质后。在另一个干燥的反应管中加入铱催化剂,菲啰啉配体,连接真空泵,在氩气氛围下置换氩气,以此加入环丁醇硅醚衍生物,四氢呋喃,氢受体。将反应管置于室温下搅拌2小时,再放入100℃油浴下反应24小时。
[0042]
5)方法一:待步骤4)反应完成后,将反应管置于78℃下冷却并加入一定量有机金属试剂,在78℃下反应3小时。其中,所述一定量的有机溶剂是指以步骤3)中手性环丁醇的用量为基准,所述一定量有机金属试剂用量为1.0-5.0mmol/mmol。
[0043]
方法二:待步骤4)反应完成后,将反应管冷却至室温,依次加入一定量的khco3,kf,h2o2,meoh,在50℃下反应10小时。其中,所述一定量的有机溶剂是指以步骤3)中手性环丁醇的用量为基准,所述一定量khco3,kf,h2o2用量都为1.0-20mmol/mmol。一定量meoh用量为1.0-5.0ml/mmol。
[0044]
结合以下具体实施例,对本发明作进一步的详细说明。实施本发明的过程、条件、实验方法等,除以下专门提及的内容之外,均为本领域的普遍知识和公知常识,本发明没有特别限制内容。所有实施例中所涉及的cbs催化剂,氢受体,菲啰啉配体的具体结构式和对应编号如下所示:
[0045][0046]
实施例1
[0047][0048]
其中,thf表示四氢呋喃,mmol表示毫摩尔,ml表示毫升,m表示摩尔每升,ee表示对映异构体过量百分数。
[0049]
往一个干燥的封管中依次加四氢呋喃(1ml),(s)-b-me(0.100mmol,0.10ml,1.0m in thf),bh3·
sme2(0.600mmol,0.30ml,2.0m in thf),使用注射泵滴加1a(0.250g,1.00mmol)的thf(1.0ml)溶液,滴加速度为1ml/h。滴加完毕后,室温搅拌1h,加入甲醇(1ml)
室温搅拌5分钟,浓缩,快速柱层析(淋洗剂:石油醚(60~90℃)/乙酸乙酯=10/1)得到手性环丁醇产物(r)-2a(0.139g,94%):固体;ee:91%(ad-h;5%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;t1=8.8min(minor);t2=9.4min(major).178.93(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.42-7.38(m,2h),7.31-7.22(m,4h),7.20-7.09(m,4h),4.12(dd,j=9.5,7.1hz,1h),3.44(dd,j=11.7,7.2hz,1h),2.49(dd,j=11.7,9.5hz,1h),1.61(brs,1h),1.13(s,3h),0.94(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):147.26,143.80,128.00,127.97,127.84,127.74,125.69,125.60,70.80,50.86,49.07,39.75,26.40,20.43.hrms-dart(m/z):[m nh4]
calcd.for c
18h24
on,270.1851;found,270.1852。
[0050][0051]
往一个干燥的封管中依次加入手性环丁醇2a(0.126g,0.500mmol)。将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,加入新鲜制备的三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时。将封管连接真空系统,真空下将多余的四氢呋喃与二乙基硅烷抽去得到硅醚中间体。在另一个封管中加入二(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)。将封管密闭好置于室温反应2小时,再放入100℃油浴下反应24小时。恢复室温,置于-78℃下并加入phli(1.2m的乙醚溶液,1.25ml,1.50mmol),在-78℃下反应3小时,加入饱和氯化铵溶液(1.0ml)淬灭反应,恢复室温加入水(5.0ml),分别用二氯甲烷(10ml),二氯甲烷(10ml),二氯甲烷(5.0ml)萃取,干燥,浓缩,快速柱层析(淋洗剂:石油醚(60~90℃)/二氯甲烷=3/1)得到手性环丁醇产物3a(56.1mg,27%):无色液体;ee:92%(ad-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=17.1min(minor);t2=26.6min(major).32.07(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.56-7.49(m,2h),7.37-7.30(m,5h),7.31-7.16(m,6h),7.17-7.04(m,2h),3.77(dt,j=7.0,5.4hz,1h),3.09(dd,j=12.6,7.0hz,1h),2.67(dd,j=12.5,5.6hz,1h),1.21(d,j=14.8hz,1h),1.13-1.06(m,s,3h oh,1h),1.04-0.87(m,9h),0.87-0.79(m,2h).
13
c nmr(101mhz,cdcl3)δ(ppm):146.24,146.01,138.17,134.33,129.07,128.65,128.07,127.82,127.78,127.59,125.47,125.39,72.93,54.80,51.75,37.31,27.67,20.25,7.64,7.48,5.20,4.79.hrms-dart(m/z):[m nh4]
calcd.for c
28h38
onsi,432.2712;found,432.2717。
[0052]
实施例2
[0053][0054]
操作同实施例1。(s)-b-me(0.200mmol,0.20ml,1.0m in thf),bh3·
sme2(1.20mmol,0.60ml,2.0m in thf),四氢呋喃(4.0ml),1b(0.252g,2.00mmol)。快速柱层析(淋洗剂:石油醚(60~90℃)/乙酸乙酯=10/1)得到手性环丁醇产物(r)-2b(0.239g,93%):白色固体;ee:95%(od-h;0%i-proh in hexanes;flow rate=2.0ml/min;detection at 210nm;t1=4.6min(major);t2=5.7min(minor)通过苯甲酸酯衍生物测定.-40.59(c1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):3.93(virt,q,j=7.6hz,1h),2.03(dd,j=11.1,7.6hz,1h),1.63(dd,j=11.1,8.2hz,1h),1.41(d,j=7.4hz,1h),0.99(s,3h),0.98(s,3h),0.95(s,3h),0.92(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):44.18,42.50,32.53,25.62,24.02,22.45,17.96.hrms-ei(m/z):[m-h2o]
calcd.for c8h
14
,110.1092;found,110.1090.
[0055][0056]
操作同实施例1。硅醚中间体的制备使用(r)-2b(64.0mg,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得3b(0.110g,68%):无色液体;ee:97%(oj-h;2%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;t1=4.1min(major);t2=7.2min(minor).7.73(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.60-7.55(m,2h),7.42-7.30(m,3h),3.54(dt,j=7.3,3.8hz,1h),1.97(dd,j=12.1,7.0hz,1h),1.41(dd,j=12.1,3.7hz,1h),1.17(d,j=14.7hz,1h),1.07-0.97(m,10h),0.97-0.86(m,2h),0.84-0.79(m,s,3h d,j=14.7hz,1h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.29,134.27,129.03,128.03,74.11,45.61,40.70,36.97,26.42,24.68,23.95,15.77,7.58,7.54,5.05,4.79.hrms-dart(m/z):[m nh4]
calcd.for c
18h34
onsi,308.2402;found,308.2404.
[0057]
实施例3
[0058][0059]
操作同实施例1。(s)-b-me(0.200mmol,0.20ml,1.0m in thf),bh3·
sme2(1.20mmol,0.60ml,2.0m in thf),四氢呋喃(2 2ml),1c(0.348g,2.00mmol)。快速柱层析(淋洗剂∶石油醚(60~90℃)/乙酸乙酯=10/1)得到一对非对映异构体手性环丁醇产物(r)-2c(0.326g,93%;cis-2c:trans-2c=1∶0.93):无色液体;两个非对映异构可以通过半制备液相色谱分离(ymc-pack sil,40%mtbe in hexanes,10.0ml/min).ee:cis-2o92%,trans-2o 98%(ad-h;2%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;cis-2o t1=14.9min(minor);t2=17.3min(major),trans-2o t1=20.4min(minor);t2=21.5min(major).(1r,3r)-2,2-dimethyl-3-phenylcyclobutan-1-ol(cis-2c).-40.94(c 1.21,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.37-7.26(m,2h),7.25-7.16(m,1h),7.16-7.08(m,2h),3.96(t,j=7.9hz,1h),2.81(dd,j=11.2,7.5hz,1h),2.52(dt,j=10.9,7.3hz,1h),2.15(td,j=11.0,8.6hz,1h),1.69(brs,1h),1.26(s,3h),0.67(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):139.80,128.04,127.42,125.96,71.90,47.02,41.66,31.81,28.32,15.66.hrms-ei(m/z):[m]
calcd.for c
12h16
o,176.1201;found,176.1196.(1r,3s)-2,2-dimethyl-3-phenylcyclobutan-1-ol(trans-2c).5.88(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.34-7.25(m,2h),7.22-7.11(m,3h),4.08(t,j=6.4hz,1h),3.25(dd,j=9.7,6.1hz,1h),2.63(ddd,j=12.6,7.4,6.1hz,1h),2.21(ddd,j=12.6,9.7,5.6hz,1h),1.72(brs,1h),1.24(s,3h),0.65(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):141.73,128.08,127.68,125.86,73.35,44.99,44.66,31.66,23.30,22.95.hrms-ei(m/z):[m]
calcd.for c
12h16
o,176.1200;found,176.1196.
[0060][0061]
操作同实施例1。硅醚中间体的制备使用cis-2c(88.1mg,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得cis-3c(79.3mg,47%):无色液体;ee:92%(od-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=14.2min(major);t2=15.3min(minor).-24.15(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.51-7.44(m,2h),7.37-7.26(m,5h),7.24-7.16(m,
in hexanes;flow rate=0.5ml/min;detection at 210 nm;t1=24.9min(minor);t2=32.2min(major).-33.60(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.12(d,j=7.8hz,2h),7.01(d,j=7.9hz,2h),3.95(dd,j=8.6,7.1hz,1h),2.77(dd,j=11.1,7.5hz,1h),2.51(dt,j=10.8,7.3hz,1h),2.34(s,1h),2.13(td,j=11.0,8.6hz,1h),1.73(brs,1h),1.25(s,3h),0.67(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):136.66,135.40,128.74,127.31,71.90,46.87,41.29,31.91,28.29,20.98,15.65.hrms-ei(m/z):[m]
calcd.for c
13h18
o,190.1350;found,190.1352.(1r,3s)-2,2-dimethyl-3-(p-tolyl)cyclobutan-1-ol(trans-2d)ee:98%(ad-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=29.1min(major);t2=30.5min(minor).2.13(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.12(d,j=7.9hz,2h),7.03(d,j=8.1hz,2h),4.08(ddd,j=7.0,5.7,1.1hz,1h),3.20(dd,j=9.8,6.0hz,1h),2.60(ddd,j=12.5,7.4,6.0hz,1h),2.33(s,3h),2.21(ddd,j=12.6,9.7,5.6hz,1h),1.76(brs,1h),1.24(s,3h),0.66(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.62,135.28,128.77,127.58,73.35,44.57,44.51,31.79,23.30,22.91,20.97.hrms-ei(m/z):[m]
calcd.for c
13h18
o,190.1355;found,190.1352.
[0067][0068]
操作同实施例1。硅醚中间体的制备使用(r)-2d(0.206g,1.08mmol),三(三苯基膦)二氯化钌(ii)(1.9mg,2.00μmol)的四氢呋喃溶液(0.2ml),二乙基硅烷(0.132g,1.50mmol),四氢呋喃溶液(0.8ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(16.8mg,25.0μmol),l1(14.2mg,60.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,nbe(0.113g,1.20mmol),四氢呋喃(4.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。快速柱层析(淋洗剂∶石油醚(60~90℃)/乙酸乙酯=20/1)得3d(0.233g,61%;cis-3d:trans-3d=1:1.08):无色液体;两个非对映异构可以通过快速柱层析(淋洗剂∶石油醚(60~90℃)/二氯甲烷=3/1)得到一部分的纯品用于表征。(1r,2s,3r)-2-((diethyl(phenyl)silyl)methyl)-2-methyl-3-(p-tolyl)cyclobutan-1-ol(cis-3d).ee:93%(ad-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=13.6min(minor);t2=15.2min(major).-26.42(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.53-7.46(m,2h),7.38-7.30(m,3h),7.12(d,j=7.8hz,2h),7.00(d,j=7.8hz,2h),3.80(td,j=7.6,5.2hz,1h),2.71(dd,j=10.9,7.6hz,1h),2.40(dt,j=11.2,7.4hz,1h),2.35(s,3h),2.04(td,j=11.0,8.0hz,1h),1.30(s,3h),1.12(d,j=5.3hz,1h),0.94-0.82(m,8h),0.84-0.69(m,3h),0.35(d,j=
15.0hz,1h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.35,136.94,135.41,134.40,128.84,128.76,127.91,127.88,72.71,49.72,44.48,30.81,29.51,21.02,13.93,7.58,7.49,5.07,4.87.hrms-dart(m/z):[m nh4]
calcd.for c
23h36
onsi,370.2561;found,370.2561.(1r,2s,3r)-2-((diethyl(phenyl)silyl)methyl)-2-methyl-3-(p-tolyl)cyclobutan-1-ol(trans-3d).ee:97%(ia;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=18.3min(major);t2=22.8min(minor).-15.00(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.65-7.55(m,2h),7.40(dd,j=4.2,2.2hz,3h),7.06(d,j=7.8hz,2h),6.86(d,j=7.8hz,2h),3.78-3.63(m,1h),3.26(t,j=8.5hz,1h),2.44(dt,j=12.5,7.3hz,1h),2.31(s,3h),2.00(ddd,j=12.9,9.3,4.0hz,1h),1.45-1.32(m,2h),1.17(d,j=14.6hz,1h),1.06-0.98(m,6h),0.97-0.91(m,4h),0.71(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.27,137.76,135.22,134.24,129.25,128.63,128.20,127.47,47.47,47.03,30.67,22.82,21.51,20.96,7.44,7.42,4.83,4.40.hrms-dart(m/z):[m nh4]
calcd.for c
23h36
onsi,370.2559;found,370.2561.
[0069]
实施例5
[0070][0071]
操作同实施例1。(s)-b-me(0.200mmol,0.20ml,1.0m in thf),bh3·
sme2(1.20mmol,0.60ml,2.0m in thf),四氢呋喃(2 2ml),1e(0.417g,2.00mmol)。快速柱层析(淋洗剂∶石油醚(60~90℃)/乙酸乙酯=10/1)得到一对非对映异构体手性环丁醇产物(r)-2e(0.326g,93%;cis-2e:trans-2e=1∶0.94):无色液体;两个非对映异构可以通过半制备液相色谱分离(ymc-pack sil,40%mtbe in hexanes,10.0ml/min).(1r,3r)-3-(4-chlorophenyl)-2,2-dimethylcyclobutan-1-ol(cis-2e).ee:92%(oj-h;3%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;t1=10.5min(minor);t2=11.3min(major).-34.80(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.32-7.22(m,2h),7.07-7.00(m,2h),3.96(q,j=7.5hz,1h),2.76(dd,j=11.1,7.4hz,1h),2.52(dt,j=10.9,7.3hz,1h),2.09(td,j=11.0,8.6hz,1h),1.59(d,j=7.3hz,1h),1.24(s,3h),0.65(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.32,131.68,128.68,128.16,71.67,47.02,41.15,31.79,28.24,15.67.hrms-ei(m/z):[m]
calcd.for c
12h15
ocl,210.0810;found,210.0806.(1r,3s)-3-(4-chlorophenyl)-2,2-dimethylcyclobutan-1-ol(trans-2e).ee:98%(oj-h;3%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;t1=10.2min(minor);t2=11.0min(major).5.77(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.34-7.20(m,2h),7.06(d,j=8.4hz,2h),4.06(t,j=6.4hz,1h),3.22(dd,j=9.7,6.2hz,1h),2.57(ddd,j=13.2,7.3,6.2hz,1h),2.21(ddd,j=12.6,9.7,5.5hz,1h),1.76(brs,1h),1.23(s,3h),0.64(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):140.23,131.56,128.95,128.20,73.19,44.66,44.46,31.60,23.24,22.87.hrms-ei(m/z):[m]
calcd.for c
12h15
ocl,210.0799;found,210.0806.
[0072][0073]
操作同实施例1。硅醚中间体的制备使用cis-2e(0.105g,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得cis-3e(0.113g,61%):无色液体;ee:92%(od-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=14.9min(major);t2=16.7min(minor).-25.00(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.54-7.45(m,2h),7.40-7.31(m,3h),7.33-7.24(m,2h),7.10-6.96(m,2h),3.80(dt,j=10.9,5.3hz,1h),2.72(dd,j=10.7,7.7hz,1h),2.42(dt,j=11.2,7.4hz,1h),2.01(td,j=10.9,7.8hz,1h),1.30(s,3h),1.15(d,j=4.5hz,1h),0.95-0.84(m,8h),0.86-0.71(m,3h),0.27(d,j=15.0hz,1h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.73,138.08,134.36,131.73,129.29,128.96,128.16,127.99,72.56,49.76,44.54,30.66,29.45,14.21,7.54,7.46,5.05,4.78.hrms-dart(m/z):[m nh4]
calcd.for c
22h33
onclsi,390.2009;found,390.2014.
[0074][0075]
操作同实施例1。硅醚中间体的制备使用trans-2e(0.105g,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得trans-3e(0.132g,71%):无色液体;ee:98%(ad-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=19.7min(major);t2=28.6min(minor).-13.35(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.65-7.56(m,2h),7.41(dd,j=4.5,1.9hz,3h),7.20(d,j=8.4hz,2h),6.83(d,j=8.3hz,2h),3.69(dd,j=6.9,3.9hz,1h),3.24(t,j=8.4hz,1h),2.41(dt,j=12.4,7.3hz,1h),2.02(ddd,j=12.8,9.2,3.9hz,1h),1.39(d,j=
14.6hz,1h brs,1h),1.15(d,j=14.5hz,1h),1.09-0.88(m,10h),0.69(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):139.87,137.56,134.25,131.45,129.33,128.81,128.25,128.00,74.18,47.25,47.19,30.58,22.77,21.48,7.40,7.38,4.82,4.33.hrms-dart(m/z):[m nh4]
calcd.for c
22h33
onclsi,390.2014;found,390.2014.
[0076]
实施例6
[0077][0078]
操作同实施例1。(s)-b-me(0.200mmol,0.20ml,1.0m in thf),bh3·
sme2(1.20mmol,0.60ml,2.0m in thf),四氢呋喃(2 2ml),1f(0.465g,2.00mmol)。快速柱层析(淋洗剂∶石油醚(60~90℃)/乙酸乙酯=10/1)得到一对非对映异构体手性环丁醇产物(r)-2f(0.448g,93%;cis-2f:trans-2f=1∶0.95):无色液体;(1r,3s)3-(2-(benzyloxy)ethyl)-2,2-dimethylcyclobutan-1-ol(cis-2f) (1r,3r)-3-(2-(benzyloxy)ethyl)-2,2-dimethylcyclobutan-1-ol(trans-2f).1h nmr(400mhz,cdcl3)δ(ppm):7.38-7.23(m,5h 5h),4.48(d,j=1.6hz,2h),4.48(s,2h),3.89(dd,j=7.1,5.3hz,1h),3.71(t,j=7.9hz,1h),3.50-3.33(m,2h 2h),2.32(dt,j=10.8,7.0hz,1h),1.99-1.78(m,4h),1.77-1.69(m,4h),1.63(d,j=15.1hz,3h),1.60-1.48(m,3h),1.41(td,j=10.5,8.7hz,1h),1.05(s,3h),1.04(s,3h),0.97(s,3h),0.93(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.47,128.33,128.32,127.59,127.49,73.26,72.96,72.94,72.26,69.10,69.04,43.83,42.02,35.22,34.74,33.84,33.22,31.27,30.12,28.02,22.27,22.10,14.98.(1r,3s)-3-(2-(benzyloxy)ethyl)-2,2-dimethylcyclobutan-1-ol(cis-2f).cis-2fwas prepared by nabh
4 reduction of 1r.1h nmr(400mhz,cdcl3)δ(ppm):7.39-7.29(m,4h),7.32-7.24(m,1h),4.48(s,2h),3.71(t,j=7.9hz,1h),3.40(t,j=6.5hz,2h),2.32(dt,j=10.8,6.9hz,1h),1.76(brs,1h),1.79-1.66(m,1h),1.64-1.48(m,1h),1.48-1.35(m,1h),1.05(s,3h),0.94(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.45,128.30,127.57,127.47,72.91,72.21,69.02,43.80,34.68,33.81,30.10,28.01,14.98.hrms-dart(m/z):[m h]
calcd.for c
15h23
o2,235.1692;found,235.1693.
[0079][0080]
操作同实施例1。硅醚中间体的制备使用(r)-2f(0.234g,1.00mmol),三(三苯基膦)二氯化钌(ii)(1.9mg,2.00μmol)的四氢呋喃溶液(0.2ml),二乙基硅烷(0.132g,1.50mmol),四氢呋喃溶液(0.8ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化
铱(i)二聚体(16.8mg,25.0μmol),l1(14.2mg,60.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(0.194g,1.00mmol),四氢呋喃(4.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。快速柱层析(淋洗剂:石油醚(60~90℃)/乙酸乙酯=10/1)得cis-3f(0.117g,58%);trans-3f(0.104g,54%):无色液体;(1r,2s,3s)-3-(2-(benzyloxy)ethyl)-2-((diethyl(phenyl)silyl)methyl)-2-methylcy clobutan-1-ol(cis-3f).ee:94%(od-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=18.4min(major);t2=22.4min(minor).18.30(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.60-7.51(m,2h),7.36-7.30(m,7h),7.30-7.22(m,1h),4.46(s,2h),3.56(t,j=7.1hz,1h),3.36(ddt,j=9.2,7.0,3.1hz,2h),2.24(dt,j=11.3,7.1hz,1h),1.81-1.65(m,1h),1.64-1.45(m,2h),1.30(ddd,j=11.2,8.7,7.2hz,1h),1.14(d,j=14.9hz,1h),1.11(s,3h),1.04-0.83(m,10h),0.79(d,j=14.6hz,1h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.51,138.27,134.38,128.93,128.30,127.97,127.59,127.46,73.37,72.90,68.87,46.11,36.95,33.11,30.97,29.07,13.66,7.62,7.56,5.17,4.96.hrms-dart(m/z):[m h]
calcd.for c
25h37
o2si,397.2553;found,397.2557.(1r,2s,3r)-3-(2-(benzyloxy)ethyl)-2-((diethyl(phenyl)silyl)methyl)-2-methylc yclobutan-1-ol(trans-3f).ee:98%(as-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=12.6min(minor);t2=13.9min(major).-22.16(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.59-7.46(m,2h),7.38-7.20(m,8h),4.42(d,j=2.7hz,2h),3.63(t,j=5.7hz,1h),3.25(td,j=8.7,5.4hz,1h),3.17(dt,j=8.9,7.0hz,1h),1.96-1.74(m,3h),1.62-1.52(m,1h),1.47(brs,1h),1.41(ddd,j=10.5,6.5,3.8hz,1h),1.06(d,j=14.5hz,1h),1.05-0.94(m,10h),0.94-0.82(m,4h).
13
c nmr(101mhz,cdcl3)δ(ppm):138.54,137.80,134.16,128.93,128.27,127.88,127.51,127.41,74.75,72.84,69.15,44.20,37.61,32.84,30.93,22.13,19.88,7.44,7.43,4.80,4.60.hrms-dart(m/z):[m nh4]
calcd.for c
25h40
o2nsi,414.2818;found,414.2823.
[0081]
实施例7
[0082][0083]
操作同实施例1。(s)-b-me(0.500mmol,0.50ml,1.0m in thf),bh3·
sme2(3.00mmol,1.5ml,2.0m in thf),四氢呋喃(5 5ml),1g(0.942g,5.00mmol)。快速柱层析(淋洗剂∶石油醚(60~90℃)/乙酸乙酯=10/1)得到一对非对映异构体手性环丁醇产物(r)-cis-2g(0.475g,50%);(r)-trans-2g(0.473g,49%):无色液体;(1r,3r)-2,2,3-trimethyl-3-phenylcyclobutan-1-ol(cis-2g).ee:97%(oj-h;3%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=17.8min(minor);t2=23.7min(major).-65.32(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.34-7.25(m,2h),
7.22-7.12(m,1h),7.11-7.03(m,2h),4.12(q,j=8.0hz,1h),2.39-2.22(m,2h),1.48(d,j=8.1hz,1h),1.33(s,3h),1.24(s,3h),0.71(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):147.90,128.01,125.95,125.50,71.26,46.40,41.29,39.43,27.54,23.21,19.37.hrms-ei(m/z):[m-h2o]
calcd.for c
13h16
,172.1246;found,172.1247.(1r,3s)-2,2,3-trimethyl-3-phenylcyclobutan-1-ol(trans-2g).ee:93%(oj-h;3%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=22.6min(major);t2=30.1min(minor).27.24(c 1.21,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.35-7.25(m,2h),7.20-7.14(m,3h),3.91(dt,j=7.0,4.2hz,1h),2.96(dd,j=12.6,7.1hz,1h),1.84(dd,j=12.5,4.3hz,1h),1.67(d,j=4.3hz,1h),1.48(s,3h),1.20(s,3h),0.68(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):147.90,127.91,126.22,125.38,72.90,45.22,44.03,38.59,27.57,26.41,18.13.hrms-ei(m/z):[m-h2o]
calcd.for c
13h16
,172.1253;found,172.1247.
[0084][0085]
操作同实施例1。硅醚中间体的制备使用cis-2g(95.2mg,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得cis-3g(48.8mg,28%):无色液体;ee:97%(ad-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=12.2min(minor);t2=16.8min(major).-34.68(c 1.21,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.54-7.46(m,2h),7.38-7.27(m,5h),7.23-7.14(m,1h),7.11-7.03(m,2h),4.00(dd,j=8.4,7.5hz,1h),2.29(dd,j=10.8,8.4hz,1h),2.18(dd,j=10.8,7.4hz,1h),1.30(s,3h),1.29(s,3h),0.98-0.81(m,8h),0.82-0.74(m,2h),0.70(d,j=14.7hz,1h),0.62(d,j=15.2hz,1h).
13
c nmr(101mhz,cdcl3)δ(ppm):147.90,138.59,134.45,128.78,128.03,127.87,126.22,125.43,71.62,49.68,42.78,38.13,28.07,23.54,18.23,7.62,7.45,5.32,5.09.hrms-dart(m/z):[m nh4]
calcd.for c
22h36
onsi,370.2557;found,370.2561.
[0086][0087]
操作同实施例1。硅醚中间体的制备使用trans-2g(95.2mg,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,
0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得trans-3g(96.1mg,55%):无色液体;(1r,2s,3s)-2-((diethyl(phenyl)silyl)methyl)-2,3-dimethyl-3-phenylcyclobutan-1-ol(trans-3s).ee:94%(oj-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=12.5min(major);t2=18.6min(minor).54.75(c 1.20,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.66-7.55(m,2h),7.40(dt,j=4.6,1.6hz,3h),7.31-7.22(m,2h),7.18-7.09(m,1h),7.08-7.01(m,2h),3.39(dd,j=6.8,1.1hz,1h),2.72(dd,j=12.7,6.8hz,1h),1.68(dd,j=14.5,1.1hz,1h),1.60(dd,j=12.7,1.1hz,1h),1.48(s,3h),1.11-0.91(m,11h),0.80(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):149.29,137.94,134.08,129.47,128.39,127.90,125.77,125.16,74.16,48.02,46.89,36.46,28.76,26.58,16.98,7.48,4.83,4.34.hrms-dart(m/z):[m nh4]
calcd.for c
22h36
onsi,370.2557;found,370.2561.
[0088]
实施例8
[0089][0090]
操作同实施例1。(s)-b-me(0.200mmol,0.20ml,1.0m in thf),bh3·
sme2(1.20mmol,0.60ml,2.0m in thf),四氢呋喃(2 2ml),1h(0.619g,2.00mmol)。快速柱层析(淋洗剂∶石油醚(60~90℃)/乙酸乙酯=20/1)得到一对非对映异构体手性环丁醇产物(r)-2h(0.326g,93%;cis-2h:trans-2h=0.96∶1):无色液体;两个非对映异构可以通过半制备液相色谱分离(ymc-pack sil,15%i-proh in hexanes,10.0ml/min).n-(((1s,3r)-3-hydroxy-1,2,2=trimethylcyclobutyl)methyl)-n,4=dimethylbenzenesulfonamide(cis-2h).ee:98%(od-h;5%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;t1=20.4min(major);t2=24.2min(minor).9.28(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.63(d,j=8.2hz,2h),7.31(d,j=8.0hz,2h),3.97(t,j=7.9hz,1h),3.13(d,j=13.3hz,1h),2.73(d,j=13.3hz,1h),2.61(s,3h),2.42(s,3h),2.07(dd,j=11.0,7.5hz,1h),1.77(dd,j=11.1,8.4hz,1h),1.70(brs,1h),1.12(s,3h),1.00(s,3h),0.95(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):143.18,134.65,129.63,127.27,71.79,55.80,45.35,41.46,35.97,35.32,22.44,21.46,20.61,17.11.hrms-dart(m/z):[m h]
calcd.for c
16h26
o3ns,312.1624;found,312.1628.
[0091]
n-(((1s,3s)-3-hydroxy-1,2,2-trimethylcyclobutyl)methyl)-n,4-dimethylbenzenesulfonamide(trans-2h).ee:92%(od-h;5%i-proh in hexanes;flow rate=1.0ml/min;derection at 210nm;t1=20.1min(minor);t2=22.7min(major).-17.10(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.64(d,j=8.3hz,
2h),7.31(d,j=8.0hz,2h),3.88(t,j=7.0hz,1h),3.05(d,j=13.4hz,1h),2.93(d,j=13.5hz,1h),2.69(s,3h),2.48(dd,j=12.1,7.4hz,1h),2.42(s,3h),1.73(brs,1h),1.59(dd,j=12.1,6.6hz,1h),1.11(s,3h),1.00(s,3h),0.95(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):143.22,134.67,129.64,127.35,72.20,56.41,45.06,39.39,37.50,37.24,22.14,21.46,20.16,18.16.hrms-dart(m/z):[m h]
calcd.for c
16h26
o3ns,312.1625;found,312.1628.
[0092][0093]
操作同实施例1。硅醚中间体的制备使用(r)-2h(0.311g,1.00mmol),三(三苯基膦)二氯化钌(ii)(1.9mg,2.00μmol)的四氢呋喃溶液(0.2ml),二乙基硅烷(0.132g,1.50mmol),四氢呋喃溶液(0.8ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(16.8mg,25.0μmol),l1(14.2mg,60.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(0.194g,1.00mmol),四氢呋喃(4.0ml)与硅醚中间体100℃油浴下反应24小时。反应液回到室温,依次加入khco3(0.250g,2.50mmol),kf(95.0mg,2.50mmol),h2o2(1.13g,10.0mmol,30%in h2o),meoh(4.0ml)。将封管密闭好置于50℃反应12小时,回到室温,加入饱和硫代硫酸钠溶液(10ml)淬灭反应,加入水(10ml),分别用乙酸乙酯(10ml),乙酸乙酯(10ml),乙酸乙酯(5.0ml)萃取,干燥,浓缩,快速柱层析(淋洗剂∶二氯甲烷/乙酸乙酯=4/1)得到cis-3h(0.102g,63%);trans-3h(94.0mg,56%):无色液体;n-(((1s,2r,3r)-3-hydroxy-2-(hydroxymethyl)-1,2-dimethylcyclobutyl)methyl)-n,4-dimethylbenzenesulfonamide(cis-3h).ee:97%(ic;20%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;t1=20.9min(minor);t2=23.3min(major).2.20(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.62(d,j=8.3hz,2h),7.30(d,j=8.0hz,2h),4.12(t,j=8.0hz,1h),3.94(d,j=11.3hz,1h),3.59(d,j=11.4hz,1h),3.19(d,j=13.3hz,1h),2.95(d,j=13-3hz,1h),2.87(brs,1h),2.63(s,3h),2.42(s,3h),2.09(d,j=8.0hz,1h),1.13(s,3h),1.01(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):143.31,134.46,129.67,127.24,72.86,72.84,65.29,54.87,49.33,42.37,36.27,35.37,21.86,21.45,17.32.hrms-dart(m/z):[m h]
calcd.for c
16h26
o4ns,328.1573;found,328.1577.n-(((1s,2r,3s)-3-hydroxy-2-(hydroxymethyl)-1,2-dimethylcyclobutyl)methyl)-n,4-dimethylbenzenesulfonamide(trans-3h).ee:93%(ic;20%i-proh in hexanes;flow rate=1.0ml/min;detection at 210 nm;t1=21.6min(major);t2=27.5min(minor).-14.33(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.67(d,j=8.2hz,2h),7.35(d,j=8.0hz,2h),4.17(d,j=11.1hz,1h),4.10(t,j=7.4hz,1h),3.54(d,j=11.0hz,1h),3.07(d,j=13.6hz,0h),2.99(d,j=13.6hz,1h),2.74(s,3h),2.60
(dd,j=12.0,7.6hz,1h),2.46(s,3h),1.83(ddd,j=12.0,7.2,1.2hz,1h),1.70(brs,1h),1.16(s,3h),1.07(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):143.39,134.57,129.72,127.39,72.42,65.58,56.85,48.32,39.27,37.76,36.41,21.49,19.14,16.56.hrms-dart(m/z):[m h]
calcd.for c
16h26
o4ns,328.1574;found,328.1577.
[0094]
实施例9
[0095][0096]
操作同实施例1。(s)-b-me(0.200mmol,0.20ml,1.0m in thf),bh3·
sme2(1.00mmol,0.5ml,2.0m in thf),四氢呋喃(2 2ml),1i(0.283g,1.00mmol)。快速柱层析(淋洗剂∶石油醚(60~90℃)/乙酸乙酯=4/1)得到一对非对映异构体手性环丁醇产物(r)-cis-2i(0.165g,56%);(r)-trans-2i(86.0mg,29%):白色固体;(1s,5s,6r)-7,7-dimethyl-3-tosyl-3-azabicyclo[3.2.0]heptan-6-ol(cis-2i).ee:44%(oj-h;15%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;t1=19.6min(minor);t2=25.4min(major).-11.72(c1.20,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.72(d,j=8.3hz,2h),7.35(d,j=8.0hz,2h),3.79(ddd,j=12.1,7.5,1.7hz,1h),3.71(d,j=9.9hz,1h),3.50(d,j=10.2hz,1h),2.99(q,j=7.2hz,1h),2.48-2.37(m,6h),2.28(d,j=12.1hz,1h),1.16(s,3h),0.98(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):143.96,130.85,129.57,128.29,73.99,49.61,47.05,44.12,40.62,38.67,30.07,21.52,14.93.hrms-dart(m/z):[m h]
calcd.for c
15h22
o3ns,296.1313;found,296.1315.(1r,5r,6r)=7,7-dimethyl-3-tosyl-3-azabicyclo[3.2.0]heptan-6-ol(trans-2i).ee:98%(oj-h;8%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;t1=21.1min(minor);t2=31.0min(major).9.82(c 1.20,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.69(d,j=8.2hz,2h),7.33(d,j=8.1hz,2h),3.78(d,j=5.0hz,1h),3.55-3.44(m,2h),2.59(dt,j=8.6,5.2hz,1h),2.51-2.44(m,2h),2.43(s,3h),2.18(t,j=8.1hz,1h),1.91(brs,1h),1.08(s,3h),1.01(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):143.64,131.79,129.52,128.05,76.89,51.61,48.29,44.03,42.25,39.38,22.93,21.78,21.52.hrms-dart(m/z):[m h]
calcd.forc
15h22
o3ns,296.1312;found,296.1315.
[0097][0098]
操作同实施例1。硅醚中间体的制备使用ent-cis-2i(0.145g,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a
(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得ent-cis-3i(0.176g,77%):无色液体;ee:69%(od-h;5%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=21.2min(minor);t2=43.6min(major).19.60(c 1.20,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.69(d,j=8.0hz,2h),7.59-7.50(m,2h),7.39-7.30(m,5h),3.64(d,j=10.0hz,1h),3.63-3.55(m,1h),3.37(d,j=10.4hz,1h),2.93(q,j=6.8hz,1h),2.46-2.34(m,5h),2.27(dd,j=10.4,7.3hz,1h),2.09-2.03(m,1h),1.15(s,3h),1.07-0.95(m,7h),0.95-0.77(m,5h).
13
c nmr(101mhz,cdcl3)δ(ppm):143.99,137.65,134.42,131.17,129.58,128.82,128.35,127.76,75.39,49.65,47.49,46.73,43.06,38.18,29.80,21.56,12.53,7.55,5.07,4.68.hrms-dart(m/z):[m h]
calcd.for c
25h36
o3nssi,458.2174;found.458.2180.
[0099][0100]
操作同实施例1。硅醚中间体的制备使用ent-trans-2i(0.145g,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得ent-trans-3i(0.181g,79%):无色液体;ee:98%(ad-h;8%i-proh in hexanes;flow rate=1.0ml/min;detection at 210nm;t1=24.1min(minor);t2=39.8min(major).-18.22(c 1.20,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.68-7.61(m,2h),7.52-7.44(m,2h),7.37-7.28(m,5h),3.65-3.56(m,1h),3.43(d,j=9.4hz,1h),3.27(d,j=10.3hz,1h),2.52-2.35(m,5h),2.20(dd,j=10.4,7.7hz,1h),2.12-2.01(m,1h),1.71(d,j=6.4hz,1h),1.10-1.02(m,s,3h d,1h),1.02-0.94(m,7h),0.90-0.82(m,4h).
13
c nmr(101mhz,cdcl3)δ(ppm):143.58,137.34,134.22,131.84,129.49,128.95,128.03,127.86,78.90,51.64,48.20,43.81,42.71,41.50,22.01,21.52,19.59,7.45,4.90,4.72.hrms-dart(m/z):[m h]
calcd.for c
25h36
o3nssi,458.2174;found,458.2180.
[0101]
实施例10
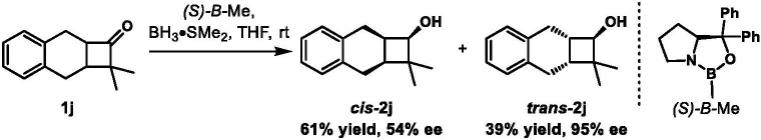
[0102][0103]
操作同实施例1。(s)-b-me(0.300mmol,0.30ml,1.0m in thf),bh3·
sme2(1.50mmol,0.75ml,2.0m in thf),四氢呋喃(3 3ml),1j(0.300g,1.50mmol)。快速柱层析
(淋洗剂∶石油醚(60~90℃)/乙酸乙酯=10/1)得到一对非对映异构体手性环丁醇产物(r)-cis-2j(0.183g,61%):白色固体;(r)-trans-2j(0.117g,39%):白色固体;(1r,2as,8ar)-2,2-dimethyl-1,2,2a,3,8,8a-hexahydrocyclobuta[b]naphthalen-1-ol(cis-2j).ee:54%(od-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=24.2min(major);t2=27.8min(minor).7.02(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.21-7.07(m,3h),3.96(d,j=7.4hz,1h),3.00-2.79(m,2h),2.76-2.56(m,3h),2.14(dtd,j=8.8,6.9,1.6hz,1h),1.43(brs,1h),1.18(s,3h),0.81(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):139.11,138.68,128.13,127.76,126.27,126.22,76.04,40.97,39.72,34.34,30.78,28.97,26.50,16.49.hrms-ei(m/z):[m]
calcd.for c
14h18
o,202.1352;found,202.1353.(1r,2ar,8as)-2,2-dimethyl-1,2,2a,3,8,8a-hexahydrocyclobuta[b]naphthalen-1-ol(trans-2j).ee:95%(oj-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=25.3min(major);t2=32.5min(minor).22.28(c 1.2,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.20-7.05(m,4h),3.27(d,j=6.8hz,1h),2.82-2.68(m,2h),2.69-2.58(m,3h),2.11(dt,j=10.5,5.6hz,1h),1.66(brs,1h),1.12(s,3h),0.84(s,3h).
13
c nmr(101mhz,cdcla)δ(ppm):139.02,138.43,128.76,128.14,126.14,125.92,76.74,40.83,39.56,37.25,31.02,29.25,23.53,21.63.hrms-ei(m/z):[m]
calcd.for c
14h18
o,202.1352;found,202.1359.
[0104][0105]
操作同实施例1。硅醚中间体的制备使用cis-2j(0.101g,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得cis-3j(0.126g,69%):无色液体;ee:96%(od-h;1%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=18.8min(minor);t2=21.6min(major).1.93(c 1.20,chcl3).1h nmr(400mhz,cdcla)δ(ppm):7.59-7.51(m,2h),7.40-7.32(m,3h),7.15-7.08(m,3h),7.10-7.02(m,1h),3.67(td,j=6.4,2.6hz,1h),2.91-2.74(m,2h),2.66(dd,j=14.2,9.0hz,1h),2.59-2.44(m,2h),2.01-1.79(m,1h),1.25(s,3h),1.11(d,j=14.7hz,1h),1.07(d,j=6.4hz,1h),1.04-0.93(m,6h),0.96-0.84(m,4h),0.81(d,j=14.7hz,1h).
13
c nmr(101mhz,cdcl3)δ(ppm):139.35,139.05,138.17,134.24,129.01,127.98,127.70,127.63,126.12,125.91,76.75,44.55,42.10,32.95,30.53,29.89,25.22,14.25,7.53,4.97,4.74.hrms-dart(m/z):[m nh4]
calcd.for c
24h36
onsi,382.2555;found,382.2561.
[0106][0107]
操作同实施例1。硅醚中间体的制备使用trans-2j(0.145g,0.500mmol),三(三苯基膦)二氯化钌(ii)(0.958mg,1.00μmol)的四氢呋喃溶液(0.1ml),二乙基硅烷(66.2mg,0.750mmol),四氢呋喃溶液(0.4ml)。将封管密闭好置于35℃反应12小时抽干。(环辛烯)氯化铱(i)二聚体(8.4mg,12.5μmol),l1(7.1mg,30.0μmol),将封管用橡皮塞塞好后,连接真空泵,在氩气氛围下置换氩气三次,在氩气保护氛围下,分别加入上述硅醚中间体,a(92.1mg,0.500mmol),四氢呋喃(2.0ml)与硅醚中间体100℃油浴下反应24小时。phli(1.2m的乙醚溶液,1.25ml,1.50mmol)在-78℃下反应3小时。柱层析得trans-3j(0.181g,79%):无色液体;ee:92%(ad-h;2%i-proh in hexanes;flow rate=0.5ml/min;detection at 210nm;t1=38.1min(minor);t2=45.5min(major).-35.82(c 1.20,chcl3).1h nmr(400mhz,cdcl3)δ(ppm):7.59-7.50(m,2h),7.40-7.32(m,3h),7.16-7.04(m,3h),7.07-6.99(m,1h),3.14(d,j=7.0hz,1h),2.75-2.60(m,2h),2.54(dddd,j=10.9,7.0,5.4,4.1hz,1h),2.45-2.29(m,2h),2.13-1.95(m,1h),1.09-0.96(m,8h),0.98-0.85(m,4h),0.80(s,3h).
13
c nmr(101mhz,cdcl3)δ(ppm):139.02,138.37,137.72,134.38,128.80,128.69,128.09,127.75,126.10,125.85,78.66,43.08,39.59,37.58,31.07,28.98,22.16,20.08,7.56,7.52,5.06,4.81.hrms-dart(m/z):[m nh4]
calcd.for c
24h36
onsi,382.2557;found,382.2561.
[0108]
上述实施例仅为了说明本发明的技术构思及特点,其目的在于让本领域技术人员能够了解本发明的内容并据以实施,并不能以此限制本发明的保护范围。凡是根据本发明内容的实质所作出的等效变化或修饰,都涵盖在本发明保护范围内。
[0109]
尽管本发明的内容已经通过上述优选实施例作了详细介绍,但应当认识到上述的描述不应被认为是对本发明的限制。在本领域技术人员阅读了上述内容后,对于本发明的多种修改和替代都将是显而易见的。因此,本发明的保护范围应由所附的权利要求来限定。
再多了解一些
本文用于创业者技术爱好者查询,仅供学习研究,如用于商业用途,请联系技术所有人。