1.本发明属于有机合成领域,具体涉及一种制备烯丙醇类化合物的方法。
背景技术:
2.nozaki-hiyama-kishi(nhk)偶联为将乙烯基-有机铬试剂添加到羰基化合物,已被证明是构建有价值的烯丙醇的有效方法。由于具有优异的化学选择性、与官能团的广泛相容性等特点,nhk反应已广泛应用于药物化学和天然产物的全合成。
3.然而,光促进cr/ni催化醛nhk烯基化产生烯丙醇仍然研究并不充分。
技术实现要素:
4.为了克服上述技术缺陷,本发明的目的在于提供了一种烯丙醇类化合物的制备方法。在镍、铬催化剂存在下,醛与三氟甲磺酸烯基酯反应,得到烯丙醇类化合物。
5.本发明所述一种烯丙醇类化合物,具体结构式如下:其中:r选自c1-c6烷基、c3-c7环烷基、叔丁基二甲基硅氧甲基、苄氧甲基、3,4-亚甲二氧基苯基、取代苯基乙基、邻苯二甲酰亚胺乙基、取代苯基、萘基、苄基、基乙基、邻苯二甲酰亚胺乙基、取代苯基、萘基、苄基、所述取代苯基中取代基为氢、卤素、腈基、硝基、三氟甲基、苯基、c1-c4烷基、c1-c4烷氧基或c1-c4烷氧羰基;x为n-boc、o、s、ch2或c(och2ch2o);m n=1-4。
6.本发明还提供了上述烯丙醇类化合物的合成方法,包括如下步骤:
7.以醛1与三氟甲磺酸烯基酯2为原料,在金属催化剂、菲罗啉类或联吡啶类配体和质子牺牲剂存在下,有机溶剂中照射反应,得到烯丙醇化合物3。反应方程式表示如下:
[0008][0009]
其中:r选自c1-c6烷基、c3-c7环烷基、叔丁基二甲基硅氧甲基、苄氧甲基、3,4-亚甲二氧基苯基、取代苯基乙基、邻苯二甲酰亚胺乙基、取代苯基、萘基、苄基、甲二氧基苯基、取代苯基乙基、邻苯二甲酰亚胺乙基、取代苯基、萘基、苄基、所述取代苯基中取代基为氢、卤素、腈基、硝基、三氟甲基、苯基、c1-c4烷基、c1-c4烷氧基或
c1-c4烷氧羰基;x为n-boc、o、s、ch2或c(och2ch2o);m n=1-4。
[0010]
进一步地,在上述技术方案中,所述金属催化剂包括镍和铬镍催化剂;镍催化剂为nibr2,铬催化剂为crcl2或crcl3。
[0011]
进一步地,在上述技术方案中,所述菲罗啉类或联吡啶类配体为菲罗啉l1、2,9-二甲基-1,10-菲罗啉l2、4,7-二苯基-1,10-菲罗啉l3或4,4-二叔丁基联吡啶l4。
[0012]
进一步地,在上述技术方案中,所述质子牺牲剂为1,4-二氢吡啶类化合物;例如:二乙基-1,4-二氢-2,6-二甲基-3,5-吡啶二羧酸酯(hant zsch酯,he)、二叔丁基-1,4-二氢-2,6-二甲基-3,5-吡啶二羧酸酯、二(甲胺基)-1,4-二氢-2,6-二甲基-3,5-吡啶二羧酸酯等。
[0013]
进一步地,在上述技术方案中,所述醛1、三氟甲磺酸烯基酯2、镍催化剂、铬催化剂、菲罗啉类配体与质子牺牲剂摩尔比为0.5:1-1.2:0.01-0.02:0.1-0.2:0.02-0.022:2-3。
[0014]
进一步地,在上述技术方案中,所述有机溶剂为乙腈、dmf、dmso或丙酮。
[0015]
进一步地,在上述技术方案中,照射反应光源为400nm led灯。
[0016]
进一步地,在上述技术方案中,反应温度为室温,反应时间为24-60小时。
[0017]
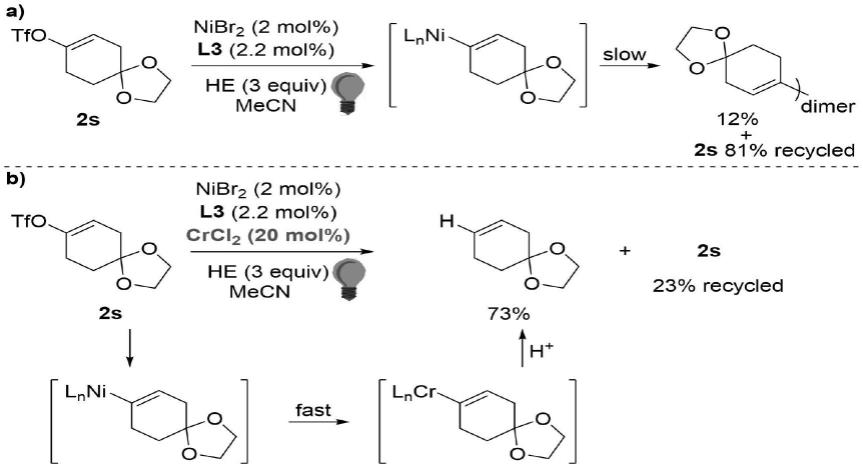
为了对反应机理进行研究,做了如下对比试验,结果采用反应方程式表示如下:
[0018][0019]
根据以上对比试验结果,推测的可能反应机理如下:
[0020][0021]
发明有益效果
[0022]
该方法具有方法操作简单,绿色环保,原料易得,反应中电子效应和位阻效应对该反应的影响小的优点。
具体实施方式
[0023]
以下结合具体实施例对本发明的技术方案作进一步详细说明,但本发明的保护范围并不局限于此。
[0024]
以1a和2a在镍、铬催化下生成3a为例,反应条件探索试验典型操作:
[0025]
氮气保护下,向nibr2(0.01mmol,0.02eq)、l3(0.011mmol,0.022eq)和无水ch3cn(0.1m)溶液中加入crcl2(0.1mmol,0.2eq),hant zsch酯(1.5mmol,3eq),1a(0.5mmol,1eq)和2a(1mmol,2eq)。将反应混合物在室温下10w400 nm led照射搅拌48小时。反应完成后,真空蒸发溶剂,粗产品快速柱色谱(石油醚/乙酸乙酯=5:1)纯化,得到无色油状化合物3a。
[0026]
反应方程式如下:
[0027]
[0028][0029]
如表所示,对反应边界条件进行了探索,发现其他可能反应条件下均以不同收率得到目标产物。最终确定最佳反应条件为:1a(0.5eq)、2a(1eq)、crcl2(20mol%)、nibr2(2mol%)、菲咯啉配体l3(2.2mol%)、he(3.0eq),在乙腈溶剂中,400nm发光二极管(led)灯照射48小时。
[0030]
实施例1:
[0031][0032]
氮气保护下,向nibr2(0.01mmol,0.02eq)、l3(0.011mmol,0.022eq)和无水ch3cn(0.1m)溶液中加入crcl2(0.1mmol,0.2eq),he(1.5mmol,3eq),1a(0.5mmol,1eq)和2a(1mmol,2eq)。将反应混合物在室温下10w400 nm led照射搅拌48小时。反应完成后,真空蒸发溶剂,粗品快速柱色谱(石油醚/乙酸乙酯=5:1)纯化,得到化合物3a:无色油状物,产率为76%(83mg)。
[0033]1h nmr(400mhz,cdcl3)δ7.29-7.25(m,2h),7.20-7.16(m,3h),5.67(dd,j=2.8,1.5hz,1h),4.15(d,j=2.8hz,2h),4.03(t,j=6.6hz,1h),3.91-3.65(m,2h),2.81-2.51(m,2h),2.21-2.13(m,1h),2.11-2.02(m,1h),1.93-1.80(m,3h).
13
c nmr(101mhz,cdcl3)δ141.75,137.80,128.37,128.35,125.82,121.43,74.48,65.15,64.17,36.18,31.81,24.02.hrms:calc’d for c
14h17
o,[m-oh]
201.1274;found 201.1271.
[0034]
实施例2:
[0035]
参考实施例1中反应条件,通过改变不同的反应底物,结果如下:
[0036]
无色油状物,产率为61%(55mg)。1h nmr(400mhz,cdcl3)δ5.63(t,j=2.3hz,1h),4.14(q,j=2.7hz,2h),3.84(dt,j=10.6,5.1hz,1h),3.76-3.71(m,2h),2.25-2.17(m,1h),2.15-1.94(m,2h),1.81(dtd,j=12.2,7.4,4.6hz,1h),1.72-1.46(m,6h),1.40(dq,j=13.0,8.1,7.6hz,1h),1.32-1.08(m,1h).
13
c nmr(101mhz,cdcl3)δ137.75,122.22,80.31,65.14,64.31,43.11,29.24,29.17,25.65,25.54,23.87.hrms:calc’d for c
11h17
o,[m-oh]
165.1274;found165.1272.
[0037]
无色油状物,产率为63%(62mg)。1h nmr(400mhz,cdcl3)δ5.61(t,j=2.3hz,1h),4.15(q,j=2.7hz,2h),3.95-3.53(m,3h),2.18(dtt,j=16.7,4.4,2.2hz,1h),2.08-1.85(m,2h),1.84-1.58(m,4h),1.58-1.36(m,2h),1.34-1.05(m,3h),1.01-0.88(m,2h).
13
cnmr(101mhz,cdcl3)δ136.97,122.48,80.35,76.68,65.20,64.28,40.47,29.58,28.57,26.40,26.13,25.93,23.99.hrms:calc’d for c
12h19
o,[m-oh]
179.1430;found 179.1427.
[0038]
无色油状物,产率为51%(52mg)。1h nmr(400mhz,cdcl3)δ5.67(ddj=2.7,1.5hz,1h),4.15(dd,j=2.6,1.0hz,2h),4.03(t,j=6.2hz,1h),3.83(dtj=10.6,5.2hz,1h),3.75(ddd j=11.2,6.7,4.6hz,1h),3.54(t,j=6.6hz,2h),2.22-2.17
(m,1h),2.15-2.12(m,1h),1.84-1.76(m,2h),1.65(h2o),1.62-1.33(m,4h).
13
c nmr(101mhz,cdcl3)δ137.77,121.52,75.06,65.16,64.19,44.87,33.73,32.38,23.87,22.92.hrms:calc’d for c
10h16
clo,[m-oh]
187.0884;found 187.0882.
[0039]
无色油状物,产率为43%(55mg)。1h nmr(400mhz,cdcl3)δ5.76(dd,j=2.8,1.5hz,1h),4.17-4.15(m,2h),4.13-4.02(m,1h),3.88-3.72(m,2h),3.67(d,j=3.6hz,1h),3.50(dd,j=9.9,8.1hz,1h),2.64(s,1h),2.21-2.13(m,1h),2.08-2.01(m,1h),1.62(h2o),1.26(petroleum ether),0.91(s,9h),0.08(s,6h).
13
cnmr(101mhz,cdcl3)δ134.35,122.28,74.68,66.04,65.28,64.12,25.84,25.05,18.27,-5.36,-5.39.hrms:calc’d for c
13h25
o2si,[m-oh]
241.1618;found 241.1614.
[0040]
无色油状物,产率为61%(91mg)。1h nmr(400mhz,cdcl3)δ5.64-5.60(m,1h),4.16-4.08(m,4h),3.83(dt,j=11.1,5.1hz,1h),3.79-3.66(m,2h),2.65(s,2h),2.29-2.13(m,1h),2.05-1.97(m,1h),1.95-1.84(m,1h),1.79(s,1h),1.63-1.53(m,1h),1.44(s,10h),1.35-1.02(m,2h).
13
c nmr(101mhz,cdcl3)δ154.79,136.48,123.19,79.52,79.31,65.12,64.19,38.95,28.56,28.42,28.01,23.85.hrms:calc’d for c
16h27
nnao4,[m na]
320.1832;found320.1826.
[0041]
无色油状物,产率为57%(67mg)。1h nmr(400mhz,cdcl3)δ7.40-7.28(m,5h),5.78(dd,j=2.8,1.5hz,1h),4.57(s,2h),4.27-4.23(m,1h),4.17-4.14(m,2h),3.89-3.69(m,2h),3.57(dd,j=9.5,3.3hz,1h),3.43(dd,j=9.6,8.2hz,1h),2.53(s,1h),2.20-2.12(m,1h),2.08-2.01(m,1h).
13
c nmr(101mhz,cdcl3)δ137.68,134.30,128.48,127.88,127.79,122.38,73.38,73.28,72.99,65.23,64.07,24.97.hrms:calc’d for c
14h17
o2,[m-oh]
217.1223;found 217.1218.
[0042]
无色油状物,产率为60%(86mg)。1h nmr(400mhz,cdcl3)δ7.47-7.35(m,4h),5.69(t,j=2.3hz,1h),4.15(d,j=2.8hz,2h),4.04(t,j=6.6hz,1h),3.82(dt j=10.7,5.2hz,1h),3.75(td j=6.6,3.3hz,1h),2.75(ddt,j=38.2,14.0,7.9hz,2h),2.23-2.14(m,1h),2.12-2.03(m,1h),1.95-1.82(m,2h),1.75(s,1h).
13
c nmr(101mhz,cdcl3)δ142.68,137.66,131.82,130.62(q,j
c-f
=31.9hz),128.76,125.09(q,j
c-f
=3.6hz),124.19(d,j
c-f
=272.7hz),122.75(q,j
c-f
=3.9hz),121.72,74.33,65.15,64.15,35.90,31.63,24.00.
19
f nmr(377mhz,cdcl3)δ-62.55.hrms:calc’d for c
15h16
f3o,[m-oh]
269.1148;found 269.1143.
[0043]
无色油状物,产率为74%(110mg)。1h nmr(400mhz,cdcl3)δ7.50-7.31(m,2h),7.18-6.95(m,2h),5.67(dd,j=2.7,1.5hz,1h),4.15(dd,j=2.7,1.0hz,2h),4.02(t,j=6.5hz,1h),3.82(dt j=10.6,5.2hz,1h),3.75(ddd j=11.2,6.7,4.6hz,1h),2.79-2.49(m,2h),2.21-2.13(m,1h),2.12-2.02(m,1h),1.93-1.75(m,2h),1.66(h2o).
13
c nmr(101mhz,cdcl3)δ140.72,137.70,131.40,130.17,121.64,119.56,76.68,74.32,65.16,64.16,35.95,31.21,24.00.hrms:calc’d for c
14h16
bro,[m-oh]
279.0379;found 279.0374.
[0044]
无色油状物,产率为70%(82mg)。1h nmr(400mhz,cdcl3)δ7.18-6.91(m,2h),6.83-6.57(m,2h),5.71-5.65(m,1h),5.36(s,1h),4.25-4.08(m,2h),4.04(t,j=6.6hz,1h),3.87-3.81(m,1h),3.79-3.73(m,1h),2.76-2.45(m,2h),2.21-2.15(m,1h),2.11-2.01(m,1h),1.90-1.77(m,2h),1.68(h2o).
13
c nmr(101mhz,cdcl3)δ153.85,137.75,133.66,129.45,121.52,115.23,74.62,65.19,64.23,36.37,30.91,24.00.hrms:calc’d for c
14h17
o2,[m-oh]
217.1223;found 217.1219.
[0045]
无色油状物,产率为40%(57mg)。1h nmr(400mhz,cdcl3)δ7.88-7.82(m,2h),7.76-7.70(m,2h),5.70(dd,j=2.7,1.5hz,1h),4.13-4.06(m,2h),4.05-3.98(m,1h),3.90-3.82(m,2h),3.81-3.69(m,2h),2.11(m,2h),2.03-1.78(m,3h).
13
c nmr(101mhz,cdcl3)δ168.76,136.96,134.09,132.00,123.33,121.22,71.76,65.17,64.11,34.61,33.52,24.45.hrms:calc’d for c
16h16
no3,[m-oh]
270.1125;found 270.1120.
[0046]
无色油状物,产率为91%(86mg)。1h nmr(400mhz,cdcl3)δ7.76-7.10(m,5h),5.88-5.84(m,1h),5.10(s,1h),4.29-4.09(m,2h),3.84-3.60(m,2h),2.19(s,1h),2.08-1.84(m,2h).
13
c nmr(101mhz,cdcl3)δ141.65,137.44,128.39,127.69,126.48,121.39,65.23,64.10,24.58.hrms:calc’d for c
12h13
o,[m-oh]
173.0961;found 173.0958.
[0047]
无色油状物,产率为71%(72mg)。1h nmr(400mhz,cdcl3)δ7.42(dd,j=7.1,2.0hz,1h),7.33-7.06(m,3h),5.76-5.73(m,1h),5.28(d,j=1.6hz,1h),4.19-4.15(m,2h),3.74(td,j=5.5,1.8hz,2h),2.32(s,3h),2.19-1.85(m,3h).
13
c nmr
(101mhz,cdcl3)δ139.43,136.57,135.62,130.45,127.53,126.18,126.03,122.00,73.60,65.26,64.15,25.12,19.11.hrms:calc’d for c
13h15
o,[m-oh]
187.1117;found 187.1114.
[0048]
无色油状物,产率为50%(67mg)。1h nmr(400mhz,cdcl3)δ7.53(dd,j=7.6,1.6hz,2h),7.34(dd,j=7.5,1.3hz,1h),7.15(dd,j=7.5,1.3hz,1h),5.83-5.81(m,1h),5.64-5.34(m,1h),4.34-4.12(m,2h),3.76(dd,j=5.4,2.1hz,2h),2.12(d,j=4.0hz,1h),2.12-2.03(m,2h),1.60(h2o).
13
c nmr(101mhz,cdcl3)δ140.53,135.81,132.82,129.22,128.35,127.61,123.21,122.76,75.25,65.30,64.11,25.11.hrms:calc’d for c
12h12
bro,[m-oh]
251.0066;found 251.0064.
[0049]
无色油状物,产率为72%(73mg)。1h nmr(400mhz,cdcl3)δ7.33-7.21(m,2h),7.20-7.08(m,2h),5.87-5.85(m,1h),5.07(s,1h),4.21-4.19(m,2h),3.75-3.71(m,2h),2.35(s,3h),2.03-1.87(m,3h).
13
c nmr(101mhz,cdcl3)δ138.75,137.52,137.46,129.12,126.49,121.15,76.87,65.28,64.12,24.75,21.09.hrms:calc’dfor c
13h15
o,[m-oh]
187.1117;found 187.1115.
[0050]
淡黄色油状物,产率为78%(104mg)。1h nmr(400mhz,cdcl3)δ7.61-7.35(m,2h),7.37-7.03(m,2h),5.82(dd,j=2.8,1.5hz,1h),5.05(s,1h),4.16(dd,j=2.7,1.3hz,2h),3.89-3.53(m,2h),2.42(s,1h),2.01-1.93(m,1h),1.91-1.84(m,1h).
13
c nmr(101mhz,cdcl3)δ140.60,137.15,131.42,128.15,121.95,121.46,76.36,65.14,64.03,24.27.hrms:calc’d for c
12h12
bro,[m-oh]
251.0066;found 251.0065.
[0051]
无色油状物,产率为65%(84mg)。1h nmr(400mhz,cdcl3)δ7.46(d,j=8.4hz,1h),7.35(d,j=2.1hz,1h),7.26(dd,j=8.4,2.1hz,1h),5.77(dd,j=2.8,1.4hz,1h),5.45(s,1h),4.15(dd,j=2.7,1.5hz,2h),3.73(td,j=5.4,1.4hz,2h),2.51(s,1h),2.03-1.96(m,2h).
13
c nmr(101mhz,cdcl3)δ137.67,135.54,133.82,133.35,129.19,128.96,127.24,122.87,72.55,65.17,64.03,24.69.hrms:calc’d for c
12h11
cl2o,[m-oh]
241.0181;found 241.0179.
[0052]
无色油状物,产率为55%(71mg)。1h nmr(400mhz,cdcl3)δ7.46(d,j=8.4hz,1h),7.35(d,j=2.1hz,1h),7.26(dd,j=8.4,2.1hz,1h),5.77(dd,j=2.8,1.4hz,1h),5.45(s,1h),4.15(dd,j=2.7,1.5hz,2h),3.73(td,j=5.4,1.4hz,2h),2.51
62.46.hrms:calc’d for c
13h12
f3o,[m-oh]
241.0835;found 241.0833.
[0058]
无色油状物,产率为62%(67mg)。1h nmr(400mhz,cdcl3)δ7.62(d,j=8.4hz,2h),7.47(d,j=8.2hz,2h),5.85(dd,j=2.7,1.5hz,1h),5.17(s,1h),4.16(dd,j=2.7,1.1hz,2h),3.80-3.59(m,2h),2.53(s,1h),2.06-1.94(m,1h),1.90-1.79(m,1h).
13
c nmr(101mhz,cdcl3)δ146.96,136.86,132.10,126.98,123.19,118.71,111.21,76.40,65.07,63.98,23.93.hrms:calc’d for c
13h12
no,[m-oh]
198.0913;found 198.0912.
[0059]
白色固体,产率为58%(72mg)。1h nmr(400mhz,cdcl3)δ8.20-7.88(m,2h),7.62-7.32(m,2h),5.85(dd,j=2.7,1.5hz,1h),5.18(s,1h),4.18(dd,j=2.6,1.2hz,2h),3.90(s,4h),3.84-3.58(m,2h),2.28(s,1h),2.05-1.97(m,1h),1.92-1.84(m,1h).
13
c nmr(101mhz,cdcl3)δ166.90,146.67,137.10,129.67,129.39,126.31,122.51,76.73,65.16,64.06,52.11,24.20.hrms:calc’d for c
14h15
o3,[m-oh]
231.1016;found 231.1015.
[0060]
无色结晶,产率为43%(60mg)。1h nmr(400mhz,cdcl3)δ5.88-5.72(m,1h),5.46(d,j=2.5hz,1h),4.18(t,j=2.7hz,2h),3.89-3.72(m,2h),2.54(s,1h),2.19-2.11(m,1h),2.04-1.95(m,1h).
13
c nmr(101mhz,cdcl3)δ146.08(m),143.46(m),138.81(m),134.34,121.89,115.07(m)77.32,77.00,76.68,68.34,65.12,63.85,25.18.
19
f nmr(377mhz,cdcl3)δ-142.70
‑‑
143.13(m),-154.36(t,j=20.8hz),-159.73
‑‑
164.42(m).hrms:calc’d for c
12
h8f5o,[m-oh]
263.0490;found 263.0489.
[0061]
淡黄色油状物,产率为72%(84mg)。1h nmr(400mhz,cdcl3)δ7.31-7.26(m,2h),7.21-7.17(m,3h),5.83(d,j=4.2hz,1h),3.99(t,j=6.7hz,1h),3.31-3.01(m,2h),2.81-2.53(m,4h),2.44-2.36(m,1h),2.27-2.19(m,1h),1.96-1.77(m,2h),1.70(s,1h).
13
c nmr(101mhz,cdcl3)δ141.71,140.80,128.35,128.32,125.80,120.01,76.23,36.26,31.94,25.27,24.93,24.47.hrms:calc’d for c
14h17
s,[m-oh]
217.1045;found 217.1042.
[0062]
无色油状物,产率为88%(120mg)。1h nmr(400mhz,cdcl3)
δ7.31-7.23(m,2h),7.21-7.11(m,3h),5.57(d,j=3.5hz,1h),4.05(t,j=6.6hz,1h),3.96(s,4h),2.75-2.67(m,1h),2.64-2.57(m,1h),2.39-2.09(m,4h),1.97-1.81(m,2h),1.75(t,j=6.5hz,2h),1.67(h2o).
13
c nmr(101mhz,cdcl3)δ142.00,139.63,128.41,128.28,125.72,120.26,108.04,75.06,64.35,36.51,35.47,32.04,30.89,22.76.hrms:calc’d for c
17h21
o2,[m-oh]
257.1536;found257.1531.
[0063]
无色油状物,产率为58%(59mg)。1h nmr(400mhz,cdcl3)δ7.39-7.24(m,2h),7.24-7.13(m,3h),5.62(d,j=2.9hz,1h),4.32-4.22(m,1h),2.77-2.68(m,1h),2.66-2.60(m,1h),2.40-2.25(m,4h),1.96-1.83(m,4h),1.61(h2o),1.53(s,1h).
13
c nmr(101mhz,cdcl3)δ146.86,142.05,128.43,128.34,125.77,125.73,70.72,37.11,32.15,31.86,31.08,23.35.hrms:calc’d for c
14h17
,[m-oh]
185.1325;found 185.1320.
[0064]
无色油状物,产率为61%(66mg)。1h nmr(400mhz,cdcl3)δ7.29(d,j=7.6hz,2h),7.24-7.07(m,3h),5.68(dt,j=5.6,2.3hz,1h),3.99(t,j=6.6hz,1h),2.74-2.66(m,1h),2.63-2.56(m,1h),2.13-1.91(m,4h),1.90-1.81(m,2h),1.71-1.35(m,5h).
13
cnmr(101mhz,cdcl3)δ142.14,139.73,128.43,128.31,125.73,123.45,76.11,36.41,32.14,24.97,23.46,22.63.hrms:calc’d for c
15h19
,[m-oh]
199.1481;found 199.1478.
[0065]
无色油状物,产率为47%(38mg)。1h nmr(400mhz,cdcl3)δ7.33-7.25(m,2h),7.24-7.16(m,3h),5.91(ddd,j=16.9,10.4,6.1hz,1h),5.25(dt,j=17.2,1.5hz,1h),5.14(dt,j=10.5,1.4hz,1h),4.13(dtt,j=6.9,5.5,1.0hz,1h),2.85-2.60(m,2h),1.94-1.77(m,2h),1.53(h2o),1.26(pe).
13
c nmr(101mhz,cdcl3)δ141.84,140.98,128.44,128.38,125.84,114.93,72.47,38.49,31.61.
[0066]
黄色固体,产率为64%(151mg)。1h nmr(400mhz,cdcl3)δ8.45-8.19(m,2h),8.02-7.81(m,2h),7.54-7.36(m,2h),7.24-7.06(m,2h),5.88-5.86(m,1h),5.14(s,1h),4.21-4.18(m,2h),3.84-3.62(m,2h),3.20-2.96(m,4h),2.36(d,j=2.9hz,1h),2.12-1.85(m,2h),1.69-1.42(m,4h),0.87(t,j=7.4hz,6h).
13
c nmr(101mhz,cdcl3)δ163.81,149.96,144.88,139.73,137.29,132.70,130.74,127.71,127.12,121.87,121.36,76.43,65.19,64.07,49.87,24.42,21.87,11.11.hrms:calc’d for c
25h31
nnao6s,[m na]
496.1764;found 496.1759.
[0067]
无色油状物,产率为47%(126mg)。1h nmr(400mhz,cdcl3)δ7.70-7.61(m,2h),7.62-7.55(m,2h),7.41-7.29(m,8h),7.12-7.04(m,2h),5.76-5.74(m,1h),5.13(s,1h),4.02-3.89(m,4h),3.29(t,j=7.0hz,2h),3.16(t,j=7.1hz,2h),2.33(d,j=3.5hz,2h),2.25(s,1h),2.18-2.10(m,1h),2.08-1.99(m,1h),1.70(t,j=6.5hz,2h).
13
c nmr(101mhz,cdcl3)δ170.63,161.43,149.82,145.53,139.77,139.12,135.12,132.34,128.88,128.61,128.52,128.48,128.06,127.85,127.46,126.49,121.26,120.88,107.95,76.79,64.33,35.54,31.22,30.75,23.47,23.31.hrms:calc’d for c
33h32
no6,[m h]
538.2224;found 538.2212.
[0068]
黄色固体,产率为56%(153mg)。1h nmr(400mhz,cdcl3)δ7.75-7.60(m,2h),7.57-7.42(m,2h),7.41-7.28(m,2h),7.15-6.96(m,3h),6.89(d,j=9.0hz,1h),6.69(dd,j=9.0,2.5hz,1h),5.84-5.81(m,1h),5.08(s,1h),4.27-4.10(m,2h),3.90(s,2h),3.83(s,3h),3.70(td,j=5.3,3.0hz,2h),2.45(s,3h),2.24(s,1h),2.07-1.82(m,2h).
13
c nmr(101mhz,cdcl3)δ169.26,168.28,156.07,150.05,139.38,139.31,137.26,136.18,133.74,131.15,130.81,130.44,129.11,127.52,121.77,121.26,114.98,111.92,111.73,101.21,76.40,65.16,64.04,55.69,30.50,24.41,13.38.hrms:calc’d for c
31h28
clnnao6,[m na]
568.1497;found 568.1492.
[0069]
无色油状物,产率为44%(109mg)。1h nmr(400mhz,cdcl3)δ7.53-7.30(m,2h),7.13-6.93(m,3h),6.77-6.50(m,2h),5.78-5.75(m,1h),5.14(s,1h),4.06-3.79(m,6h),2.38-2.32(m,2h),2.31(s,3h),2.18(s,3h),2.15-2.10(m,1h),2.08-2.01(m,1h),1.90-1.83(m,4h),1.70(t,j=6.5hz,2h),1.37(s,6h).
13
c nmr(101mhz,cdcl3)δ176.35,156.84,150.23,139.48,139.17,136.44,130.31,127.42,123.59,121.29,120.86,120.72,111.93,107.96,76.84,67.76,64.35,42.40,37.12,35.56,30.77,25.24,25.12,23.34,21.37,15.77.hrms:calc’d for c
30h38
nao6,[m na]
517.2566;found 517.2550.
[0070]
白色固体,产率为52%(157mg)。1h nmr(400mhz,cdcl3)δ7.64-7.56(m,2h),7.39-7.31(m,4h),7.15-7.08(m,2h),7.01-6.94(m,2h),6.94-6.88(m,2h),6.20(t,j=5.9hz,1h),5.75-5.73(m,1h),5.12(s,1h),4.02-3.82(m,4h),3.64(q,j=6.6hz,2h),2.86(t,j=6.9hz,2h),2.32(d,j=3.5hz,2h),2.21(s,1h),2.16-2.08(m,1h),2.04-1.96(m,1h),1.73(s,6h),1.68(t,j=6.5hz,2h).
13
c nmr(101mhz,cdcl3)δ172.90,166.36,154.07,149.71,140.06,139.08,137.55,132.90,132.65,129.58,128.75,128.21,127.50,120.97,120.93,119.39,107.91,79.21,76.73,64.32,41.21,35.53,34.68,30.74,25.40,23.24.hrms:calc’d for c
34h36
clnnao7,[m na]
628.2073;found 628.2061.
[0071]
以上所述仅是本发明的优选实施方式,应当指出,对于本技术领域的普通技术人员来说,在不脱离本发明原理的前提下,还可以做出若干改进和润饰,这些改进和润饰也应视为本发明的保护范围。
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。
